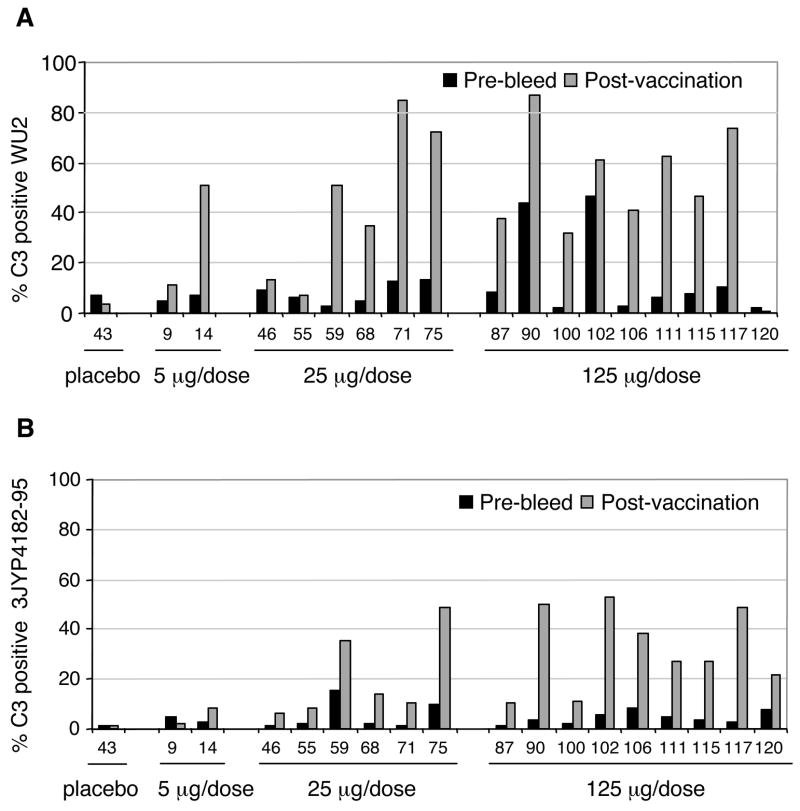
Figure 4. Comparison of C3 deposition on pneumococcal surface with pre- and post-immune human sera from subjects vaccinated with Rx1 protein.
Sera were collected from subjects who received the placebo control or Rx1 protein at immunization doses of 5 μg/dose, 25 μg/dose and 125 μg/dose. Pneumococcal strains WU2 (PspA clade 2, panel A) and 3JY4182-95 (PspA clade 1, panel B) were incubated with 10% heat-inactivated pre- or post-vaccination human sera followed by incubation with baby rabbit complement. The bars showing percent of bacteria labeled with C3 were each based on 10,000 bacteria observed by flow cytometry. Thus, the bars are highly precise and the differences between pre- and post-sera were statistically significant in every case except for those few where neither the pre-or post-immune sera mediated complement deposition on more than 15% of bacteria. Using a Wilcoxon matched-pairs signed-ranks test we did pair-wise comparisons on complement deposition mediated by pre- and post-immune sera from immunizations with 25 μg and 125 μg recombinant Rx1 PspA. For both panels A and B, the greater complement deposition by the post-immune sera was significant at P < 0.0001 by the Wilcoxon matched-pairs signed-rank test.