Figure 2.
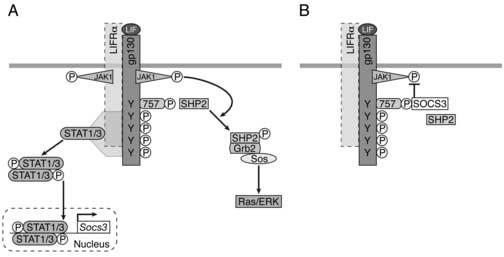
SOCS3 negatively regulates signalling via gp130. A) Binding of LIF to the multimeric receptor complex of LIFRα and gp130 results in transphosphorylation of constitutively associated JAK family kinases, leading to phosphorylation of receptor tyrosines and activation of two major signalling cascades: STAT1/3 and SHP2-Ras-ERK-MAPK. STAT1 and STAT3 bind to phosphorylated tyrosines on gp130 and LIFRα, become phosphorylated, form dimers and translocate to the nucleus where they bind DNA and activate target genes including SOCS3. Receptor-bound SHP-2 is tyrosine phosphorylated by JAKs, binds to Grb2, which is constitutively associated with the GDP/GTP Ras-exchange protein SOS, and GTP-bound Ras triggers ERK-MAPK activation. B) SOCS3 binds pTyr 757 on gp130, inhibiting JAK kinase activity and downregulating STAT1/3 signalling. In addition, SOCS3 may promote proteosomal degradation of ligand-occupied receptor complexes. SHP-2, which also binds to pTyr 757, is outcompeted by SOCS3 binding to this residue, resulting in attenuation of ERK-MAPK signalling. In the absence of SOCS3, there is prolonged activity of both pathways. For simplicity, signalling via LIFRα is not shown.
