Abstract
Pulmonary arteries (PAs), particularly those of the rat, demonstrate a prominent voltage-gated K+ (Kv) current (Ikv), which plays an important role in the regulation of the resting potential. No detailed characterization of electrophysiological and pharmacological properties of Ikv, particularly in resistance PA myocytes (PAMs), has been performed. The aim of the present study was therefore to compare Ikv in rat conduit and resistance PAMs using the standard patch clamp technique. We found that 67 % of conduit PAMs demonstrated a large, rapidly activating Ikv which was potently blocked by 4-aminopyridine (4-AP; IC50, 232 μm), but was almost insensitive to TEA (18 % block at 20 mm). Thirty-three percent of cells exhibited a smaller, more slowly activating Ikv which was TEA sensitive (IC50, 2.6 mm) but relatively insensitive to 4-AP (37 % block at 20 mm). These currents (termed Ikv1 and Ikv2, respectively) inactivated over different ranges of potential (V0.5 =−20.2 vs. -39.1 mV, respectively). All resistance PAMs demonstrated a large, rapidly activating and TEA-insensitive K+ current resembling Ikv1 (termed Ikvr), but differing significantly from it with respect to 4-AP sensitivity (IC50, 352 μm), activation rate, and inactivation potential range (V0.5, −27.4 mV). Thus, cells from conduit PAMs fall into two populations with respect to functional Ikv expression, while resistance arteries uniformly demonstrate a third type of Ikv. Comparison of the properties of the native Ikv with those of cloned Kv channel currents suggest that Ikv1 and Ikvr are likely to be mediated by Kv1.5-containing homo/heteromultimers, while Ikv2 involves a Kv2.1 α-subunit.
Work over the past decade has revealed that smooth muscle cells from different vascular beds vary widely in their functional expression of different types of K+ current (Nelson & Quayle, 1995). Within this diverse spectrum, pulmonary arteries (PAs), particularly those of the rat, are distinguished by the presence of a prominent voltage-gated K+ (Kv) current (Ikv), which plays an important role in the regulation of the resting potential in this tissue (Smirnov et al. 1994; Yuan et al. 1998). It has previously been demonstrated that native Ikv in PAMs is diminished by hypoxia (Post et al. 1992) and it was proposed that this effect may contribute to hypoxic pulmonary vasoconstriction (Weir & Archer, 1995).
In freshly isolated rat PA myocytes (PAMs) Ikv appears as a rapidly activating slowly decaying delayed rectifier with no transient component (Smirnov et al. 1994; Smirnov & Aaronson, 1994; Patel et al. 1997; Turner & Kozlowski, 1997). The current is only slightly attenuated by 3–10 mm tetraethylammonium (TEA) (Smirnov et al. 1994; Patel et al. 1997), but is markedly blocked by 4-aminopyridine (4-AP) (Smirnov et al. 1994; Yuan, 1995; Hulme et al. 1999) with an apparent dissociation constant of ∼200 μm (Smirnov & Aaronson, 1994).
Using RT-PCR and Western blot analysis and immunocytochemistry both gene and protein expression for various types of Kv α-subunits, including Kv1.1, Kv1.2, Kv1.3, Kv1.4, Kv1.5, Kv1.6, Kv2.1, Kv9.3, Kv3.1b and Kv4.3, have previously been demonstrated in rat intact pulmonary arteries and primarily cultured PAMs (Patel et al. 1997; Archer et al. 1998; Yuan et al. 1998; Hulme et al. 1999; Osipenko et al. 2000; Platoshyn et al. 2001). Kv1.4 and Kv4.3 α-subunits, which encode rapidly inactivating A-type currents (Stühmer et al. 1989; McIntosh et al. 1997), are unlikely to be responsible for the native delayed rectifier current in freshly isolated PAMs. Recently, it has been shown that the currents through several types of cloned Kv α-subunits expressed in mammalian cell lines were reduced by acute hypoxia. Thus, Patel et al. (1997) showed that the properties and hypoxia sensitivity of the native current in resistance PAMs were mimicked by co-expression of cDNA for Kv2.1 and Kv9.3 (an electrically silent Kv α-subunit) in COS cells (Patel et al. 1997). It was subsequently found that the expression of Kv1.2, as well as the co-expression of Kv1.2 and Kv1.5 α-subunits, in mouse L cells also resulted in hypoxia-sensitive currents (Hulme et al. 1999). On the other hand, Osipenko et al. (2000) reported that the expression of the Kv3.1b, but not Kv1.2, α-subunit in L929 cells resulted in the development of a hypoxia-sensitive current. These four cloned Kv channel subtypes, which encode slowly inactivating delayed rectifier currents, were therefore proposed as potential candidates for the native Ikv current inhibited by hypoxia in intact PA cells (Coppock et al. 2001), although the exact role of Kv channels in general and each individual Kv α-subunit in particular in hypoxic pulmonary vasoconstriction remains controversial (Ward & Aaronson, 1999; Coppock et al. 2001).
In addition to the molecular diversity of Kv α-subunits expressed in PAMs, the distribution of K+ channels along the pulmonary arterial tree is also not uniform. It has been shown previously that the distribution of some K+ currents, in particular the Ca2+-activated K+ current (IK,Ca), varies along the PA tree (Albarwani et al. 1995; Archer et al. 1996). The presence of three cell subtypes which differed in the relative proportion of IK,Cavs. Ikv components in the whole-cell current has been proposed in rat conduit PAMs (Archer et al. 1996). When isolated and visualized using light microscopy, these groups of cells were also found to differ with respect to their appearance. Based on this analysis the authors concluded that the relative proportion of cells containing Ikv current was greater in resistance PAMs compared with conduit PAMs (Archer et al. 1996). No detailed electrophysiological or pharmacological characterization of the whole-cell membrane currents, in particularly Ikv in resistance arteries was performed.
The multiple expression of various Kv channel subtypes in conduit and resistance PAs (Coppock et al. 2001) forms a potential basis for heterogeneity of the native Kv currents in various regions of the PA tree, although evidence for this has not been presented previously. The aim of the present study was therefore to compare Ikv in rat conduit and resistance PAs, in order both to examine whether this current exhibits regional heterogeneity, and to determine the extent to which the characteristics of Ikv correspond to those of any known Kv subtypes.
Here for the first time we present electrophysiological and pharmacological evidence which demonstrates the presence of three subpopulations of smooth muscle cells in the PA tree, each expressing a distinct Ikv in a relatively homogeneous manner. The properties of these native Ikv are compared with those previously described for cloned Kv channels, and the possible molecular correlates of Ikv in rat PA are discussed. Part of this work has been published in abstract form (Smirnov et al. 2000, 2001).
METHODS
Materials
All chemicals, enzymes for cell isolation and K+ channel inhibitors were purchased from BDH Merck (UK) or Sigma unless otherwise indicated.
Cell isolation procedure
Male Wistar rats (weight, 225–300 g) were killed by cervical dislocation in accordance with UK Home Office guidelines and the heart and lungs were removed. Conduit (main extralobar) and resistance (4th or 5th order intralobar) branches of PA were dissected, cleaned of connective tissue and cut open longitudinally. Tissues were left on ice for 30 min in normal physiological salt solution (PSS) and then transferred to nominally Ca2+-free PSS containing 0.2 mm EGTA and incubated for 10 min at 37 °C. After incubation arteries were transferred into 2 ml prewarmed nominally Ca2+-free PSS containing 2–3 or 1–2 mg ml−1 collagenase (Type XI) and 1 or 0.5 mg ml−1 papain for conduit and resistance PA, respectively. Dithiothreitol (DTT, 1 mm) was added to activate papain. Also, to increase the activity of collagenase, 10 μl normal PSS was added per 1 ml of the enzyme-containing solution, giving an estimated Ca2+ concentration of ∼15 μm. Pieces of arteries were then incubated for 25 (conduit) or 15 min (resistance) at 37 °C. After incubation tissue was transferred into Ca2+-free PSS containing 0.2 mm EGTA, cooled on ice for 5 min and then gently triturated in three sequential volumes (4 ml) of cooled Ca2+-free EGTA-containing PSS. The last two volumes were combined and cells were spun down at 1100 g and then resuspended in cooled Ca2+-free PSS (0.3–0.5 ml). The final volume of the cell suspension was then doubled using normal PSS. The cell suspension was stored on ice and cells were used on the same day. Single smooth muscle cells used for electrophysiological recordings had an elongated spindle shape and were able to contract reversibly upon the approach of the patch pipette filled with high K+ solution containing ATP. For Western blot analysis conduit and resistance PAs were pooled from at least four animals to obtain a sufficient quantity of the total protein.
Electrophysiological experiments
Current recordings were made with an Axopatch 200B patch clamp amplifier and pCLAMP 8.02 software (Axon Instruments, Foster City, CA, USA) using the whole-cell configuration of the patch clamp technique. Currents were filtered at 2 kHz and sampled at 5 kHz. Experiments were performed at room temperature. Electrode resistance when filled with the pipette solution was 1–5 MΩ, which gave a mean series resistance in whole-cell mode of 11 ± 0.4 MΩ (n = 85) and 12 ± 1 MΩ (n = 29) in conduit and resistance PAMs, respectively. Since no significant difference in series resistance between the three groups of cells described in Results was found, this was not compensated. At the beginning of each experiment, the capacitative transient in response to 10 mV hyperpolarizing step depolarization (filtered at 50 kHz and sampled at 200 kHz) was recorded. The cell membrane capacitance (Cm) was then calculated from the area under the capacitative transient. Holding potential was −80 mV.
Immunoblot analysis
Cell lysates were prepared in lysis buffer containing a protease inhibitor cocktail (‘Complete’, Boehringer Mannheim). Tissues were homogenized using an Ultra-Turrax T25 homogenizer (Janke & Kunkel, IKA-Labortechnik, 24 000 r.p.m., 2 × 1 min, 4 °C) and then agitated slowly for 1 h at 4 °C. Debris was removed by centrifugation (2 500 g, 30 min, 4 °C) and supernatants were used immediately or stored at −70 °C.
Samples (20–50 μg protein) were mixed with SDS-gel loading buffer and separated using 6 or 8 % acrylamide gels. The total protein concentration was measured by the Bradford method using bovine serum albumin as a standard protein, and an equal amount of protein was loaded for each gel. Proteins were blotted onto PVDF membranes, and then washed in phosphate buffered saline (PBS, Gibco) and PBS containing 0.05 % Tween-20 (PBST). After blocking in a 5 % (w/v) solution of dried skimmed milk in PBST for 1 h at room temperature, membranes were probed overnight (4 °C) with either Kv1.2 (1 : 1000), Kv1.5 (1 : 500), Kv2.1 (1 : 1000) or Kv3.1b (1 : 500) antibodies (Alomone Labs, Israel) in 1 % milk in PBST. Proteins were labelled with a secondary horseradish peroxidase-conjugated goat anti-rabbit antibody (1 : 2000) for 1 h at room temperature. Bound antibody was detected by the ECL method (Amersham). All bands observed were due to specific binding of the corresponding anti-Kv antibody, as they were not detected when the primary antibody was pre-incubated (blocked) with the corresponding antigen (data not shown).
During preliminary work we found that the fraction of protein which is recognized by the Kv1.5 antibody was sensitive to boiling or even preheating of protein samples to 60 °C for 10 min, a standard procedure used to denature the protein before loading on gel (data not shown). This effect was also observed with Kv1.5 antibodies obtained from Upstate Biotechnology or Chemicon. Other Kv antibodies used in this study were virtually unaffected by high temperature. To minimize the possible degradation of the Kv1.5 channel protein and increase sensitivity of Kv1.5 antibodies, the samples were therefore not heated before loading.
Solutions
PSS contained (mm): 130 NaCl, 5 KCl, 1.5 CaCl2, 1.2 MgCl2, 10 Hepes and 10 glucose, pH was adjusted to 7.2 with NaOH. Nominally Ca2+-free solution was obtained by omitting CaCl2 from PSS. The pipette solution contained (mm): 110 KCl, 10 NaCl, 5 MgATP, 10 Hepes, 10 EGTA and 0.5 CaCl2 (giving an estimated free [Ca2+] of 8 nm); the pH was adjusted to 7.2 with KOH. Lysis buffer had the following composition (mm): 50 Tris-Cl (pH 7.5), 250 NaCl, 5 EDTA, 5 DTT, 10 NaF and 0.1 % (v/v) Igepal.
Curve fitting and statistical analysis
Data analysis, curve fitting and presentation were performed using pCLAMP 8.02 (Axon Instruments, Foster City, CA, USA) and Origin 6.0 (Microcal Software, Northampton, MA, USA) software. The results are expressed as means ± s.e.m. Student's t test was used to calculate the statistical significance of the differences between two populations. Values of P < 0.05 were considered to indicate significance unless stated otherwise.
RESULTS
Kv channel currents in rat conduit pulmonary arteries
The voltage-gated K+ current (Ikv) was recorded in normal PSS in the presence of 1 μm paxilline and 10 μm glibenclamide in order to eliminate any residual contamination by BKCa and/or KATP channel currents, respectively. Under these conditions, it quickly became apparent that two types of Ikv were present in the rat conduit PA, and that individual cells predominantly expressed either one or the other. The two types of current are illustrated in Fig. 1A and B. In the majority of cells (39/66), the current was of high amplitude (1574 ± 146 pA at +60 mV) and activated rapidly (Fig. 1A). In the minority of cells (27/66), the current activated more slowly, and was much smaller (308 ± 41 pA; P < 0.0001) (Fig. 1B). The difference in amplitude was not due to cell size, since the membrane capacitance was not significantly different between two groups of cells (16.5 ± 1.2 pF, n = 39, and 19.7 ± 1.8 pF, n = 27, respectively). Moreover, we found that the larger current showed very little sensitivity to TEA, with even 20 mm causing little block. On the other hand, in cells with the smaller, slower activating Ikv, the current was mostly blocked by 20 mm TEA (compare Fig. 1A and B).
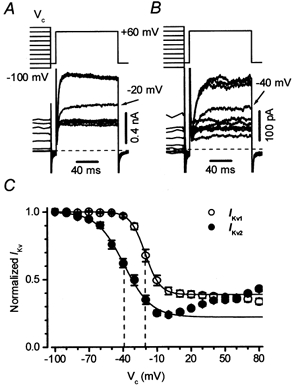
Figure 1. Two subpopulations of cells in rat conduit PA.
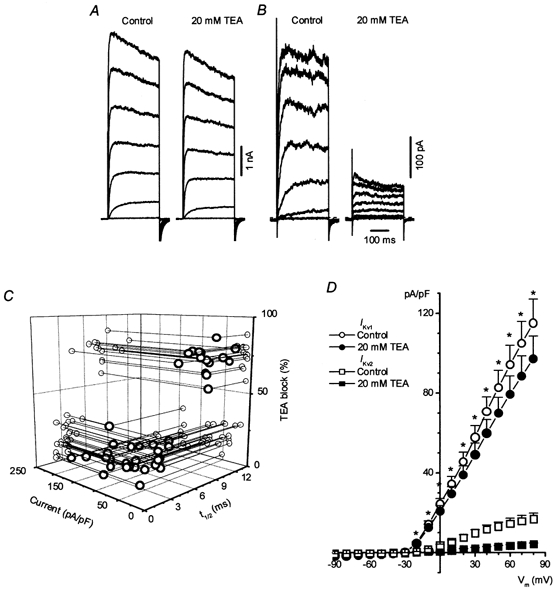
A and B, families of Ikv recorded from two representative cells in response to a 300 ms membrane depolarization between −40 and +80 mV in 20 mV increments and the effect of 20 mm TEA on the current. Holding potential was −80 mV. Cm = 6.3 (A) and 14.4 pF (B). Here and in subsequent figures horizontal dashed lines indicate zero current level. C, a relationship between the current density, percentage of TEA (20 mm) block and half-time to maximum Ikv amplitude (t1/2) in 59 rat conduit PAMs. All parameters were measured at the test potential of +60 mV. Lines and grey circles show the projection of individual data points on corresponding planes. D, I-V relationships for Ikv1 (n = 26) and Ikv2 (n = 7) in two groups of cells in the absence and presence of 20 mm TEA as indicated. Asterisks show significant differences between Ikv1 and Ikv2 densities under both conditions (P < 0.003).
In Fig. 1C, the relationship between the percentage of block by 20 mm TEA has been plotted against current density and also t1/2, the time taken for the current to attain 50 % of its maximal amplitude, in 59 cells in which all of these parameters were measured with steps to +60 mV. It is clear that the currents fall into two groups on the basis of each of these characteristics. The groups could be particularly well distinguished on the basis of their sensitivity to TEA, since currents were either > 55 % or < 35 % inhibited by 20 mm TEA; no cells were found which demonstrated an intermediate TEA block.
In the group (n = 39) which was more sensitive to 20 mm TEA (> 55 % block), the mean t1/2 was 7.4 ± 0.4 ms, the mean current density was 17 ± 1.3 pA pF−1, and the mean current amplitude was 268 ± 31 pA. In the group which was relatively insensitive to TEA (< 35 % block), the mean t1/2 was 3.6 ± 0.2 ms, the mean current density was 103 ± 10 pA pF−1, and the current amplitude was 1416 ± 147 pA (n = 27). Each of these parameters was highly significantly different in the two groups of cells (P < 0.003). Figure 1D depicts the current-voltage (I-V) relationship, and the effect of 20 mm TEA, in these two groups of cells. TEA blocked the current significantly in both groups (P < 0.0001 at +60 mV), but the extent of the block differed greatly between the groups over a wide range of potentials. Hereafter, the relatively TEA-insensitive rapidly activating currents will be referred to as Ikv1, and the TEA-sensitive slowly activating currents will be termed Ikv2.
Figure 2A and B illustrates the concentration dependency of the block by TEA of the current at +60 mV in a cell demonstrating Ikv2. The current was approximately halved by 3 mm TEA in this cell, and recovered quickly even after the approximately 80 % block achieved at 20 mm TEA. The IC50 for TEA was 2.6 ± 0.2 mm in a group of 6 cells of this type.
Figure 2. Effect of TEA on Ikv in rat conduit PAMs.
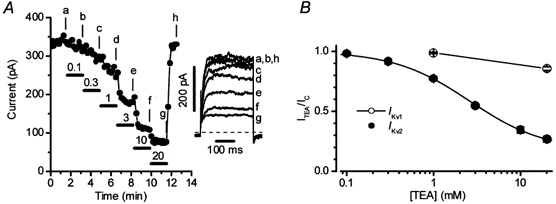
In 26 cells, in which Ikv1 predominated, the current was not sensitive to a low concentration (1 mm) of TEA but was inhibited by 18 ± 2.4 % by 20 mm TEA (Fig. 2B, open circles, see also Fig. 1D). One explanation of this effect of 20 mm TEA was that some Ikv2 was also present in these cells and was being blocked by the drug. This possibility was supported by the fact that the mean absolute amplitude of the TEA-sensitive component was very similar, regardless of whether Ikv1 or Ikv2 predominated in a particular cell (Fig. 1D). Furthermore, block of Ikv1 by 4-AP (see below) typically revealed a small current with the pharmacological and kinetic properties of Ikv2 (i.e. block by TEA, inactivation over more negative potentials, a slower activation). We did not, however, attempt to characterize this current in any detail due to preliminary experiments which suggested that its properties were somewhat altered by the presence of 4-AP. Due to the presence of this current, subsequent experiments designed to characterize Ikv1 were carried out in the presence of 20 mm TEA to minimize a possible contamination by Ikv2. It should be noted, however, that parallel experiments were also usually carried out in the absence of TEA, and always gave similar results (see below), probably because a contribution of Ikv2 to the net Kv current in Ikv1 cells was very small in comparison to that of Ikv1. The converse possibility, that Ikv1 was significantly contaminating the current in ‘Ikv2 cells’, also seemed unlikely, as described below.
Figure 3A shows an example of the block by 4-AP of Ikv2 in a cell in which this current predominated. It is apparent that Ikv2 was relatively insensitive to 4-AP. Figure 3B shows an experiment in which the effect of 4-AP on Ikv1 was evaluated in the presence of 20 mm TEA. 4-AP blocked Ikv1 with a higher efficacy and potency than it demonstrated against Ikv2, as also shown for groups of cells in Fig. 3C. The IC50 for block of Ikv1 by 4-AP (in the presence of 20 mm TEA, open circles) was 232 ± 35 μm, while Ikv2 was only blocked by 37 ± 3 % (n = 5) at 20 mm 4-AP, the highest concentration tested (open squares). The block of Ikv1 by 4-AP which was observed in the absence of TEA (filled circles) was similar to that seen in its presence.
Figure 3. Inhibition of Ikv by 4-aminopyridine in rat conduit PAMs.
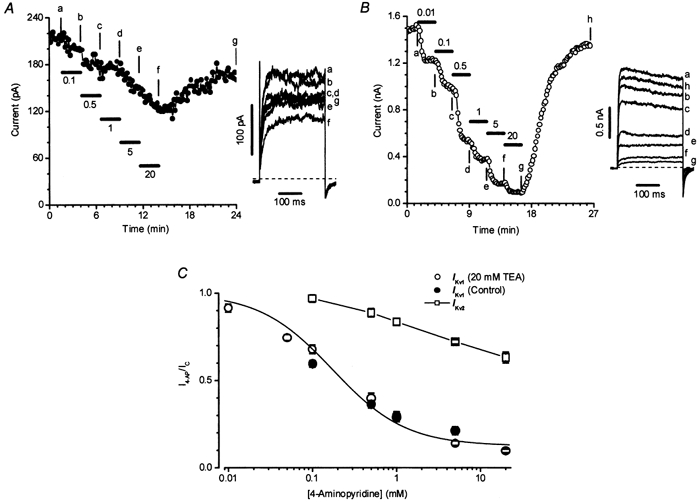
A and B, the effect of various concentrations of 4-AP (indicated in mm) on Ikv2 and Ikv1 in two representative cells, respectively, using the experimental protocol described in the legend to Fig. 2A. Insets show current traces at times indicated by the letters. Cm was 12.7 (A) and 12 pF (B). C, concentration dependence of the block of Ikv1 (circles) and Ikv2 (squares) by 4-AP. The continuous line through the circles is drawn according to the equation described in the legend to Fig. 2B with an IC50 of 0.17 mm and a residual component A equal to 0.12. Open (n = 11) and filled (n = 6) circles show normalized current in the presence and absence of 20 mm TEA, respectively. The line through the squares (n = 6) is drawn by eye. I4-AP/IC represents a ratio of the current in the presence of the corresponding concentration of 4-AP (I4-AP) and control current (IC) in the absence of the inhibitor.
It is noteworthy that 0.1 mm 4-AP produced a substantial inhibition of Ikv1, but had virtually no effect on the current in Ikv2 cells. This suggested that any contamination by Ikv1 of the current in Ikv2 cells was likely to be negligible.
In both Ikv1 and Ikv2 cells, the kinetics of activation of the outward current could be well fitted by a single exponential function over wide range of potentials, as shown in Fig. 4A. Figure 4B demonstrates that the resulting time constants of activation of Ikv2 were significantly greater than those for Ikv1 at all test potentials between −20 and +80 mV. Moreover, the presence of 20 mm TEA had no significant effect on the time constant for Ikv1 activation at any potential. In contrast to the time constant of activation, the voltage dependency of the steady-state activation of the outward current (calculated according to the Boltzmann equation described in the legend to Fig. 4) was very similar for both Ikv1 and Ikv2 cells, and was also not affected by 20 mm TEA in Ikv1 cells.
Figure 4. Activation of Ikv1 and Ikv2 in rat conduit PAMs.
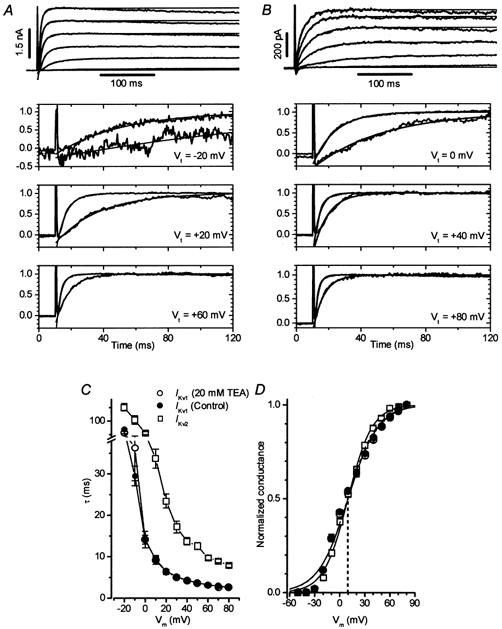
The voltage dependency of current availability in Ikv1 and Ikv2 cells was assessed by stepping cells to +60 mV, following 10 s steps to conditioning potentials ranging from −100 to +80 mV. Examples of typical experiments are shown in Fig. 5A (for Ikv1) and 5B (for Ikv2), and the mean current availabilities derived from a number of similar experiments are depicted in Fig. 5C. Ikv2 inactivated to a greater extent, and over a more negative potential range compared with Ikv1. When the data were fitted with the Boltzmann function, V0.5, the potential at which the current was half-inactivated, was −39.1 ± 2.3 mV (n = 13) in Ikv2 cells, and at the maximal extent of inactivation was 77 %. In contrast, V0.5 was −20.2 ± 1.6 mV in Ikv1 (n = 13, P < 0.0001) cells, and the maximal level of inactivation was 62 % (P < 0.0001). Moreover, as the conditioning potential was made progressively more positive, the current in Ikv2 cells began to increase again. This type of U-shaped current availability profile was never observed in Ikv1 cells.
Figure 5. Inactivation of Ikv1 and Ikv2 in rat conduit PAMs.
Kv channel currents in resistance pulmonary arterial myocytes
Myocytes isolated from resistance PAs were significantly smaller (Cm, 8.2 ± 0.5 pF, n = 29, P < 0.0001) than those from the total of 85 conduit PAMs studied with either Ikv1 (15.4 ± 0.9 pF, n = 57) or Ikv2 (19.9 ± 1.8 pF, n = 28). Figure 6A illustrates a typical family of voltage-gated K+ currents in a resistance artery cell. The current (hereafter Ikvr) resembled that present in Ikv1 cells of the conduit PA (Fig. 6B) both in its rapid rate of activation, and in its low sensitivity to 20 mm TEA (18 ± 2.7 %, n = 22 for Ikvrvs. 18 ± 2.4 %, n = 26, for Ikv1). Similar to Ikv1, Ikvr was not blocked by 1 mm TEA (not shown). Also, the mean Ikvr amplitude (0.18 ± 0.4 nA, n = 23) measured at +60 mV in PSS was not significantly different from that for Ikv1 (0.16 ± 0.15 nA, n = 39). The smaller cell size in the resistance arteries resulted, however, in a significant difference in the current density between Ikv1 and Ikvr both in the absence and presence of 20 mm TEA (Fig. 6C). No significant differences in the cell slope resistance, calculated from a linear range of I-V relationship between −90 and −70 mV, between Ikvr (11 ± 3 GΩ, n = 23) and Ikv1 (13 ± 3 GΩ, n = 39) or Ikv2 (8 ± 1 GΩ, n = 27) were found.
Figure 6. Voltage-gated K+ currents in rat resistance PAMs.
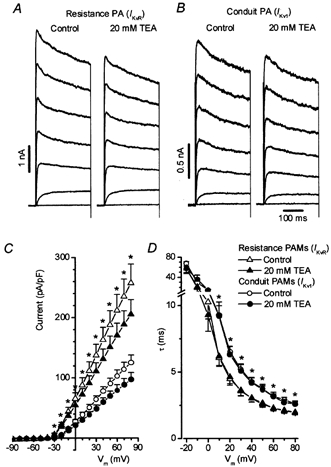
A, a family of Ikv recorded from the cell isolated from resistance PA in the presence and absence of 20 mm TEA (Cm = 14.2 pF). For comparison Ikv1 recorded under similar conditions in conduit PA cell is also shown in B (Cm = 19.4 pF). C and D compare I-V relationships for Ikvr and Ikv1 densities (C) and the dependence of the time constant of current activation (τ) on the membrane potential (Vm, D) in the absence and presence of 20 mm TEA in 23 and 22 myocytes (Ikvr) and 39 and 26 myocytes (Ikv1), respectively. Asterisks show significant differences (P < 0.003 in C and P < 0.05 in D).
When examined more closely, in addition to its higher current density, Ikvr exhibited properties clearly differentiating it from Ikv1. The time constant of activation of Ikvr was significantly larger than that of Ikv1 at potentials ranging from +10 to +80 mV (Fig. 6D), a property which was observed in both the presence and absence of 20 mm TEA. Also, as summarized in Table 1, both the activation and inactivation of Ikvr occurred over a potential range which was about 7 mV more negative than that found for Ikv1. Finally, it also emerged that compared with Ikv1, Ikvr was blocked by 4-AP with a significantly lower potency (IC50 = 352 ± 27 vs. 232 ± 35 μm, Table 1). In contrast to the conduit PAMs, no heterogeneity in the Kv current characteristics was found in the 29 resistance PAMs studied.
Table 1.
Comparison of electrophysiological and pharmacological properties of Ikvr and Ikv1
| Resistance PAMs (Ikvr) | Conduit PAMs (Ikv1) | P | ||
|---|---|---|---|---|
| Activation parameters | V0.5 (mV) | 2.9 ± 1.8 | 9.9 ± 1.4 | < 0.003 |
| k (mV) | 17.6 ± 0.4 | 17.3 ± 0.3 | n.s. | |
| (n = 26) | (n = 26) | |||
| Inactivation parameters | V0.5 (mV) | −27.4 ± 2 | −20.2 ± 1.7 | < 0.02 |
| k (mV) | 5.9 ± 0.6 | 5.7 ± 0.5 | n.s. | |
| A | 0.32 ± 0.02 | 0.38 ± 0.02 | < 0.004 | |
| (n = 13) | (n = 13) | |||
| 4-AP sensitivity | IC50 (μM) | 352 ± 27 | 232 ± 35 | < 0.009 |
| B | 0.13 ± 0.02 | 0.1 ± 0.01 | n.s. | |
| (n = 9) | (n = 11) |
Comparison was performed in the presence of 20 mm TEA to block a TEA-sensitive component of the whole-cell Ikv. n.s., not significant. A is the non-inactivating component of the current. B is a residual 4-AP-insensitive component of Ikv.
Effect of α-dendrotoxin on Kv currents in rat conduit and resistance PAMs
It is known that α-dendrotoxin (DTX) is a potent inhibitor of number of homomultimeric Kv channels which belong to the Kv1 subfamily. Low doses of DTX block Kv channels encoded by Kv1.1, Kv1.2 or Kv1.6 α-subunits (IC50, 4–20 nm), but not by the Kv1.5 α-subunit (IC50 > 1 μm) (Harvey, 2001). This selective effect of α-dendrotoxin makes this toxin a useful pharmacological tool which allows its use as a probe for the presence of Kv1.1, Kv1.2 or Kv1.6 homomultimeric currents in native cells. The effect of α-dendrotoxin on Ikvr, Ikv1 and Ikv2 in rat PA cells was therefore investigated (Fig. 7). As can be seen from the figures, the currents recorded in representative Ikv1, Ikv2 and Ikvr cells at +60 mV in the absence and 5 min after addition of 200 nm α-dendrotoxin were practically superimposable. The average inhibition of Ikv was equal to 1.5 ± 1.4 (n = 7), 2.5 ± 2 (n = 5) and 4.4 ± 4.5 % (n = 6) for Ikv1, Ikv2 and Ikvr, respectively, suggesting that the native currents in rat PAMs are not sensitive to α-dendrotoxin.
Figure 7. Effect of α-dendrotoxin on Ikv in rat resistance and conduit PAMs.
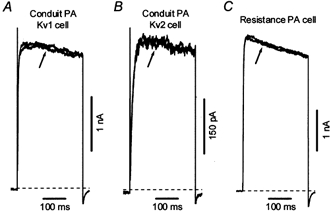
Ikv were recorded at +60 mV in the absence and 5 min after addition of 200 nm α-dendrotoxin (at arrows) in Kv1 (A, Cm = 38.3 pF) and Kv2 (B, Cm = 21.6 pF) cells isolated from conduit PAs and in resistance PAMs (C, Cm = 8.6 pF).
Expression of Kv1.2, Kv1.5, Kv2.1 and Kv3.1b α-subunits in rat conduit and resistance PAs
Figure 8 illustrates immunoblots for Kv1.2, Kv1.5, Kv2.1 and Kv3.1b channels carried out on homogenates from conduit and resistance pulmonary arteries. Channel proteins for Kv1.2, Kv1.5 and Kv2.1, but not Kv3.1b, were detected in both sizes of arteries.
Figure 8.
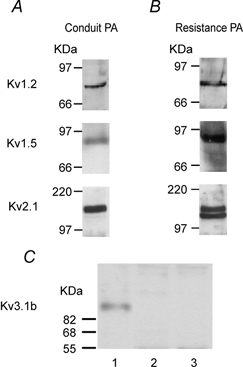
Expression of Kv1.2, Kv1.5 and Kv2.1 α-subunits in rat conduit (A) and resistance (B) PAs. C shows the Western blot analysis of the expression of Kv3.1b α-subunit in rat brain (lane 1), which was used as a positive control, in comparison to conduit (lane 2) and resistance (lane 3) PA.
DISCUSSION
In this study we have for the first time demonstrated a heterogeneity of Kv channel currents in the rat PA vasculature. About 67 % of the 85 conduit PAMs studied demonstrated a large TEA-insensitive and 4-AP-sensitive current termed Ikv1, whereas a relatively small TEA-sensitive and 4-AP insensitive current, designated Ikv2, was recorded in the remaining 33 % of conduit PA cells. In addition to differences in pharmacological characteristics, these currents were also distinct in their kinetics of activation and voltage dependence of inactivation. In contrast, every resistance PA cell we studied contained a large current (Ikvr) which resembled Ikv1, but which could be unambiguously distinguished from it on both pharmacological and biophysical grounds.
The presence of three cell types in rat conduit PA which differ in the relative expression of Ikv and IK,Ca were reported previously by Archer et al. (1996). Using TEA as a Ca2+-activated K+ current (IK,Ca) blocker, and 4-AP as a Ikv inhibitor, they found that in the majority of rat conduit PAMs (70 %, referred to as ‘mixed’ cells) the net outward current was partially blocked by both TEA (5–10 mm) and 4-AP (4 mm), whereas equal minorities of cells expressed either mainly TEA-sensitive (IK,Ca predominant) or 4-AP-sensitive (Ikv predominant) components. Cells containing only IK,Ca were ∼3 times larger than those containing only Ikv, and the ‘mixed’ cells were of intermediate capacitance. When isolated and visualized using light microscopy, these groups of cells were also found to differ with respect to their appearance and based on this morphological analysis the authors concluded that the relative proportion of cells containing Ikv current (65 %) was greater in resistance PAMs compared with conduit PAMs (Archer et al. 1996). We did not, however, detect any obvious morphological dissimilarity between PAMs dominated by Ikv1, Ikv2 or Ikvr and, although Ikv2 cells tended to be bigger than Ikv1 cells, this difference in Cm was not significant. Our direct electrophysiological measurement of whole-cell currents also indicated a relative homogeneity of cell population in resistance arteries, at least with respect to Kv channel currents. Moreover, our observation that Ikv2 was only slightly inhibited by 5 mm 4-AP, but was greatly blocked by TEA with an IC50 of 2.6 mm, suggests that the pharmacological approach of using high concentrations of TEA to distinguish Ca2+-activated and voltage-gated K+ currents employed by Archer et al. (1996) was flawed, so that cells predominantly expressing the TEA-sensitive voltage-gated K+ current (Ikv2 cells in conduit PA) could have been incorrectly classified as containing ‘mixed’ or Ca2+-activated K+ currents.
The exact molecular identity of Kv channel genes which are responsible for the native Ikv in pulmonary arteries remains inconclusive although recent evidence strongly suggest that Kv1.2, Kv1.5, Kv2.1 and Kv3.1b α-subunits are the most likely candidates (Coppock et al. 2001). Our immunoblot analysis demonstrated that Kv1.2, Kv1.5 and Kv2.1 α-subunits were expressed in both conduit and resistance arteries isolated from rat. In addition, comparison of the pharmacological and biophysical profile of Ikv1, Ikv2 and Ikvr with those reported for the delayed rectifier types of cloned Kv channels suggests that the Kv1.5 channel profile corresponds closely to that of Ikv1 and Ikvr, whereas Ikv2 mimics the properties of the Kv2.1 channel (Table 2). Homomultimers of Kv1.1, Kv1.2 and Kv1.6 are unlikely to be responsible for Ikv1 and Ikvr, since these currents were insensitive to high doses of DTX (200 nm) and only slightly sensitive to TEA (20 mm), observations which are similar to those described previously for Kv currents in rat resistance PAs (Patel et al. 1997). The presence of the Kv1.2 α-subunit protein in both conduit and resistance arteries is consistent with the possibility that Ikv1 and Ikvr could be encoded by Kv1.2- Kv1.5 heteromultimers. It has been shown previously that Kv1.2-Kv1.5, but not Kv1.1-Kv1.5 (Hatton et al. 2001), heteromultimers become insensitive to DTX and also CTX (Russell et al. 1994; Hulme et al. 1999).
Table 2.
Comparison of electrophysiological and pharmacological properties of the native Kv currents in rat PAMs and cloned Kv channels
| Ikv1, Ikvr | Ikv2 | Kv1.1a,b | Kv1.2a,b | Kv1.5a,b | Kv1.6c | Kv2.1d | Kv3.1ba | |
|---|---|---|---|---|---|---|---|---|
| Rate of activation | Rapid | Slow | Rapid | Rapid | Rapid | Rapid | Slow | Rapid |
| U-shape of inactivation | No | Yes | No | No | No | No | Yese | No |
| TEA† | > 20 | 2.6 | 0.3–0.6 | > 200 | >200 | 4 | 3–10 | 0.1–0.2 |
| 4-AP† | 0.23; 0.35 | >20 | 0.29–1 | 0.6–0.8 | 0.17–0.5 | 0.3 | 5–100 | 0.03–0.1 |
| DTX‡ | > 200 | > 200 | 12–20 | 4–17 | > 1000 | 9–25 | n.d. | > 1000 |
Superscript letters indicate the following references:
Klemic et al. (1998), Kerschensteiner & Stocker (1999). n.d., not determined.
Values are given as IC50 in mm or nm, respectively.
An important functional contribution of Kv3.1b to Ikv in rat PAMs seems to be ruled out, since when expressed this current is potently blocked by micromolar doses of both TEA and 4-AP (Table 2). This pharmacological profile does not match any of the three types of currents we observed, since we found that 1 mm TEA had no effect on Ikv1 and Ikvr currents and Ikv2 current was relatively insensitive to 4-AP. Also, Western blot analysis showed the expression of the Kv3.1b α-subunit in rat brain, which was used as a positive control, but not in resistance or conduit arteries (Fig. 8C).
It is noteworthy that under conditions when IK,Ca was eliminated using high Ca2+ buffered pipette solution and the specific IK,Ca inhibitor paxilline, a small TEA-sensitive current component in cells expressing predominantly Ikv1 and Ikvr was present. Although, as described in Results, this current was difficult to characterize, it is possible that it represents a component of Ikv2 which is constitutively present in all PAMs. This suggestion is also supported by the presence of Kv2.1 α-subunit proteins in resistance PA, where no Ikv2 cells were detected. This question, however, needs to be further addressed with inhibitors other than 4-AP, which could selectively eliminate Ikv1 or Ikvr without alteration of their properties. The presence of this small TEA-sensitive current, however, did not affect our characterization of the properties of Ikv1 and Ikvr, since no significant differences between the electrophysiological and pharmacological properties of these currents were found in the absence and presence of 20 mm TEA.
The divergence in properties between Ikv1 and Ikvr is intriguing, and could arise from a different heteromultimeric combination of, for example, Kv1.5 and Kv1.2 α-subunits, or the formation of more complex oligomeric channels composed of more than one channel protein (Po et al. 1993; Shamotienko et al. 1997). Alternatively, the association of different types of Kv β-subunits with Kv α-proteins in rat conduit and resistance PA could be responsible for the differences in the properties of Ikv1 and Ikvr. It has been found that co-expression of Kv β1 and Kv β2 subunits can both modulate the voltage dependence of activation and inactivation (McCormack et al. 1995; Uebele et al. 1996; McIntosh et al. 1997), and modify the pharmacological sensitivity (Shi et al. 1996) of Kv1 α-subunits. Although mRNA for all three subtypes of Kv β-subunits was shown to be expressed in rat PA cells (Yuan et al. 1998; Platoshyn et al. 2001) and the expression of Kv β proteins increased in resistance in comparison to conduit bovine PAMs (Coppock et al. 2001), identification of the origin of these differences will require further experimental work.
Since it was first demonstrated that Kv currents in PAMs were diminished by a reduction in PO2 (Post et al. 1992), Kv channels have been thought to act as an oxygen sensor in PA (Weir & Archer, 1995). Although, recent work from our laboratory suggests that the contribution of Ikv to acute hypoxic pulmonary vasoconstriction is limited (Robertson et al. 2000), Ikv in rat PAMs was significantly diminished by chronic hypoxia, a condition which is associated with sustained membrane depolarization and increased cell size (Smirnov et al. 1994; Barnes & Liu, 1995; Osipenko et al. 1998). In support of these data in a recent elegant study Platoshin et al. (2001) using quantitative RT-PCR showed that Kv1.5, Kv2.1 and Kv9.3 gene expression and Kv1.5 and Kv2.1 α-protein expression were significantly reduced in PAs but not mesenteric arterial cells grown under hypoxic conditions in primary culture (Platoshyn et al. 2001). These data suggest that diminution of Kv channels could be an important contributor to long-term PA depolarization, the mechanisms of which remain in dispute (Barnes & Liu, 1995). We therefore believe that our findings could form a functional basis for elucidation of these mechanisms.
Acknowledgments
This work was supported by the British Heart Foundation (grants BS/950001, PG/96151 and FS/2000013).
REFERENCES
- Albarwani S, Heinert G, Turner JL, Kozlowski RZ. Differential K+ channel distribution in smooth muscle cells isolated from the pulmonary arterial tree of the rat. Biochemical and Biophysical Research Communications. 1995;208:183–189. doi: 10.1006/bbrc.1995.1321. [DOI] [PubMed] [Google Scholar]
- Archer SL, Huang JMC, Reeve HL, Hampl V, Tolarova S, Michelakis E, Weir EK, Huang JM. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circulation Research. 1996;78:431–442. doi: 10.1161/01.res.78.3.431. [DOI] [PubMed] [Google Scholar]
- Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercure JV, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1. 5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. Journal of Clinical Investigation. 1998;101:2319–2330. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ, Liu SF. Regulation of pulmonary vascular tone. Pharmacological Reviews. 1995;47:87–131. [PubMed] [Google Scholar]
- Coppock EA, Martens JR, Tamkun MM. Molecular basis of hypoxia-induced pulmonary vasoconstriction: role of voltage-gated K+ channels. American Journal of Physiology — Lung Cellular and Molecular Physiology. 2001;281:L1–12. doi: 10.1152/ajplung.2001.281.1.L1. [DOI] [PubMed] [Google Scholar]
- Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Auperin DD, Chandy KG. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1. 1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Molecular Pharmacology. 1994;45:1227–1234. [PubMed] [Google Scholar]
- Grupe A, SchrÖter KH, Ruppersberg JP, Stocker M, Drewes T, Beckh S. Cloning and expression of a human voltage-gated potassium channel. A novel member of the RCK potassium channel family. EMBO Journal. 1990;9:1749–1756. doi: 10.1002/j.1460-2075.1990.tb08299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AL. Twenty years of dendrotoxins. Toxicon. 2001;39:15–26. doi: 10.1016/s0041-0101(00)00162-8. [DOI] [PubMed] [Google Scholar]
- Hatton WJ, Mason HS, Carl A, Doherty P, Latten MJ, Kenyon JL, Sanders KM, Horowitz B. Functional and molecular expression of a voltage-dependent K+ channel (Kv1. 1) in interstitial cells of Cajal. Journal of Physiology. 2001;533:315–327. doi: 10.1111/j.1469-7793.2001.0315a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme JT, Coppock EA, Felipe A, Martens JR, Tamkun MM. Oxygen sensitivity of cloned voltage-gated K+ channels expressed in the pulmonary vasculature. Circulation Research. 1999;85:489–497. doi: 10.1161/01.res.85.6.489. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner D, Stocker M. Heteromeric assembly of Kv2. 1 with Kv9.3: effect on the state dependence of inactivation. Biophysical Journal. 1999;77:248–257. doi: 10.1016/S0006-3495(99)76886-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch GE, Drewe JA, Verma S, Brown AM, Joho RH. Electrophysiological characterization of a new member of the RCK family of rat brain K+ channels. FEBS Letters. 1991;278:55–60. doi: 10.1016/0014-5793(91)80082-e. [DOI] [PubMed] [Google Scholar]
- Klemic KG, Shieh CC, Kirsch GE, Jones SW. Inactivation of Kv2. 1 potassium channels. Biophysical Journal. 1998;74:1779–1789. doi: 10.1016/S0006-3495(98)77888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack K, McCormack T, Tanouye M, Rudy B, StÜhmer W. Alternative splicing of the human Shaker K+ channel b1 gene and functional expression of the b2 gene product. FEBS Letters. 1995;370:32–36. doi: 10.1016/0014-5793(95)00785-8. [DOI] [PubMed] [Google Scholar]
- McIntosh P, Southan AP, Akhtar S, Sidera C, Ushkaryov Y, Dolly JO, Robertson B. Modification of rat brain Kv1. 4 channel gating by association with accessory Kv b1.1 and b2.1 subunits. Pflügers Archiv. 1997;435:43–54. doi: 10.1007/s004240050482. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. American Journal of Physiology. 1995;268:C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Osipenko ON, Alexander D, MacLean MR, Gurney AM. Influence of chronic hypoxia on the contributions of non-inactivating and delayed rectifier K currents to the resting potential and tone of rat pulmonary artery smooth muscle. British Journal of Pharmacology. 1998;124:1335–1337. doi: 10.1038/sj.bjp.0702006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipenko ON, Tate RJ, Gurney AM. Potential role for Kv3. 1b channels as oxygen sensors. Circulation Research. 2000;86:534–540. doi: 10.1161/01.res.86.5.534. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Lazdunski M, HonorÉ E. Kv2. 1/Kv9.3, a novel ATP-dependent delayed-rectifier K+ channel in oxygen-sensitive pulmonary artery myocytes. EMBO Journal. 1997;16:6615–6625. doi: 10.1093/emboj/16.22.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin LJ, Yuan X-J. Chronic hypoxia decreases KV channel expression and function in pulmonary artery myocytes. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2001;280:L801–812. doi: 10.1152/ajplung.2001.280.4.L801. [DOI] [PubMed] [Google Scholar]
- Po S, Roberds S, Snyders DJ, Tamkun MM, Bennett PB. Heteromultimeric assembly of human potassium channels. Molecular basis of a transient outward current? Circulation Research. 1993;72:1326–1336. doi: 10.1161/01.res.72.6.1326. [DOI] [PubMed] [Google Scholar]
- Post JM, Hume JR, Archer SL, Weir EK. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. American Journal of Physiology. 1992;262:C882–890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- Robertson TP, Hague D, Aaronson PI, Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. Journal of Physiology. 2000;525:669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SN, Overturf KE, Horowitz B. Heterotetramer formation and charybdotoxin sensitivity of two K+ channels cloned from smooth muscle. American Journal of Physiology. 1994;267:C1729–1733. doi: 10.1152/ajpcell.1994.267.6.C1729. [DOI] [PubMed] [Google Scholar]
- Shamotienko OG, Parcej DN, Dolly JO. Subunit combinations defined for K+ channel Kv1 subtypes in synaptic membranes from bovine brain. Biochemistry. 1997;36:8195–8201. doi: 10.1021/bi970237g. [DOI] [PubMed] [Google Scholar]
- Shi G, Kleinklaus AK, Marrion NV, Trimmer JS. Properties of Kv2. 1 K+ channels expressed in transfected mammalian cells. Journal of Biological Chemistry. 1994;269:23204–23211. [PubMed] [Google Scholar]
- Shi G, Nakahira K, Hammond S, Rhodes KJ, Schechter LE, Trimmer JS. β subunits promote K+ channel surface expression through effects early in biosynthesis. Neuron. 1996;16:843–852. doi: 10.1016/s0896-6273(00)80104-x. [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Aaronson PI. Alteration of the transmembrane K+ gradient during development of delayed rectifier in isolated rat pulmonary arterial cells. Journal of General Physiology. 1994;104:241–264. doi: 10.1085/jgp.104.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov SV, Beck R, Aaronson PI. Evidence for two populations of cells with different voltage-gated K+ currents in rat conduit pulmonary arteries. Journal of Physiology. 2000;526:P132–133P. [Google Scholar]
- Smirnov SV, Beck R, Tammaro P, Aaronson PI. Differing voltage-gated K+ currents in rat conduit and resistance pulmonary arteries. Biophysical Journal. 2001;80:214a. doi: 10.1113/jphysiol.2001.013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov SV, Robertson TP, Ward JPT, Aaronson PI. Chronic hypoxia is associated with reduced delayed rectifier K+ current in rat pulmonary artery muscle cells. American Journal of Physiology. 1994;266:H365–370. doi: 10.1152/ajpheart.1994.266.1.H365. [DOI] [PubMed] [Google Scholar]
- StÜhmer W, Ruppersberg JP, SchrÖter KH, Sakmann B, Stocker M, Giese KP, Perschke A, Baumann A, Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO Journal. 1989;8:3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R, Marshall J, Smith JS, Williams JB, Boyle MB, Folander K, Antanavage J, Oliva C, Buhrow SA, Bennett C, Stein RB, Kaczmarek LK. Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron. 1990;4:929–939. doi: 10.1016/0896-6273(90)90146-7. [DOI] [PubMed] [Google Scholar]
- Turner JL, Kozlowski RZ. Relationship between membrane potential, delayed rectifier K+ currents and hypoxia in rat pulmonary arterial myocytes. Experimental Physiology. 1997;82:629–645. doi: 10.1113/expphysiol.1997.sp004052. [DOI] [PubMed] [Google Scholar]
- Uebele VN, England SK, Chaudhary A, Tamkun MM, Snyders DJ. Functional differences in Kv1. 5 currents expressed in mammalian cell lines are due to the presence of endogenous Kv b2.1 subunits. Journal of Biological Chemistry. 1996;271:2406–2412. doi: 10.1074/jbc.271.5.2406. [DOI] [PubMed] [Google Scholar]
- Ward JP, Aaronson PI. Mechanisms of hypoxic pulmonary vasoconstriction: can anyone be right? Respiration Physiology. 1999;115:261–271. doi: 10.1016/s0034-5687(99)00025-0. [DOI] [PubMed] [Google Scholar]
- Weir EK, Archer SL. The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels. FASEB Journal. 1995;9:183–189. doi: 10.1096/fasebj.9.2.7781921. [DOI] [PubMed] [Google Scholar]
- Yuan X-J. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circulation Research. 1995;77:370–378. doi: 10.1161/01.res.77.2.370. [DOI] [PubMed] [Google Scholar]
- Yuan X-J, Wang J, Juhaszova M, Golovina VA, Rubin LJ. Molecular basis and function of voltage-gated K+ channels in pulmonary arterial smooth muscle cells. American Journal of Physiology. 1998;274:L621–635. doi: 10.1152/ajplung.1998.274.4.L621. [DOI] [PubMed] [Google Scholar]


