Figure 2. CNS stem cells differentiate into smooth-muscle-like cells at low density.
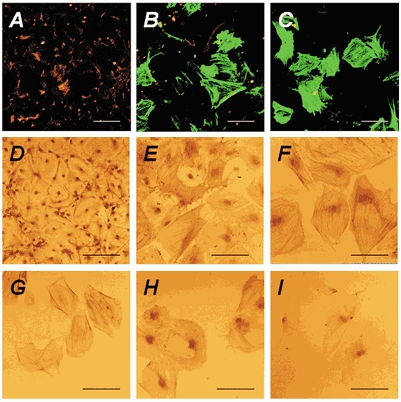
CNS stem cells were cultured on poly-l-ornithine-fibronectin-coated glass coverslips at various cell densities as described in Methods. Undifferentiated CNS stem cells were then cultured for 7 days with 10 % FBS after bFGF withdrawal. After differentiation, the cultures were subjected to immunocytochemistry. A-C, anti-GFAP and anti-α-smooth-muscle actin antibodies followed by secondary antibodies conjugated to R-PE (red; for GFAP) or fluorescein (green; for α-smooth muscle actin) were used for double-labelling immunocytochemistry, respectively. D-I, cells were incubated with anti-GFAP (D), anti-α-smooth muscle actin (E, F), anti-SMemb (G), anti-SM-1 (H) and anti-SM-2 (I), followed by biotinylated anti-mouse secondary antibodies. Virtually all of the cells at the highest density (1 × 105 cells cm−2) were immunoreactive for GFAP (red in A and D), whereas no α-smooth-muscle-actin-immunoreactive cells were detected (A). Decreasing the cell density to 5 × 103 cells cm−2 decreased the number of GFAP-immunoreactive cells, with a concomitant increase in the number of α-smooth-muscle-actin-immunoreactive cells (B, E). All of the cells at low density (2.5 × 103 cells cm−2) were immunoreactive for α-smooth muscle actin (green in C, F), SMemb (G), SM-1 (H) and SM-2 (I) myosin heavy chains, but not for GFAP (red in C, F). Most of the cells at low density displayed a flattened morphology and α-smooth-muscle-actin-labelled stress fibres (F). Scale bars represent 100 μm.
