Figure 4. Time-dependent contraction of collagen gels.
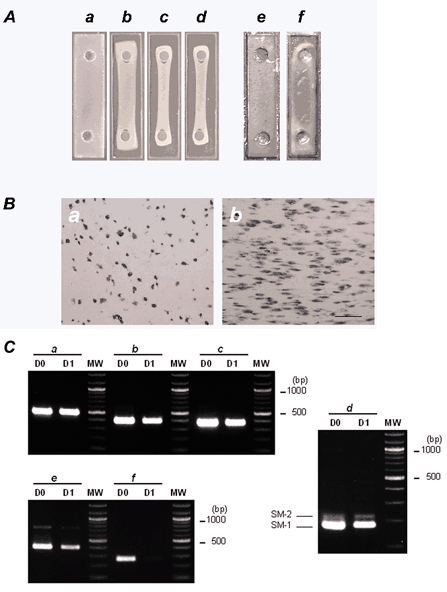
A, photographs showing time-dependent contraction of collagen gels. A collagen gel suspension containing 2.5 × 106 cells ml−1 of CNS stem-cell-derived smooth-muscle-like cells was poured into a rectangular well and placed in a CO2 incubator at 37 °C in a humidified 5 % CO2-95 % air atmosphere. The collagen gel suspension gelled within 30 min (a). The preparations were incubated in N2 medium in the presence of 10 % FBS for 3 days. Shown are photographs of the gel suspension after 8 h (b), 1 day (c) and 3 days of incubation (d). One day after gels were cast, the diameter of the cross-section was markedly reduced and formed a string-shaped fibre. A collagen gel suspension containing 2.5 × 106 cells ml−1 of CNS stem-cell-derived astrocytes was incubated for 3 days. e, before incubation; f, after 3 days incubation. B, haematoxylin-eosin staining of longitudinal sections of collagen gels before incubation (a) and after 1 day of incubation (b). Longitudinal sections were cut using a cryostat and subjected to haematoxylin-eosin staining. Bar indicates 100 μm and represents the direction of the isometric axis. C, RT-PCR analysis of total RNAs extracted from collagen gels before incubation (D0) and after 1 day of incubation (D1). RT-PCR was performed as described in Methods using primers specific for GAPDH (a), α-smooth muscle actin (b), SM-22 (c), SM-1/SM-2 myosin heavy chains (d), basic calponin (e) and nestin (f). The number of PCR cycles was as follows: GAPDH, × 25; α-smooth muscle actin, × 25; SM-22, × 27; SM-1/SM-2 myosin heavy chains, × 35; basic calponin, × 29; nestin, × 29. The PCR products were resolved by electrophoresis on 2 % agarose (3 % in the case of SM-1/ SM-2 myosin heavy chains) prestained with ethidium bromide. No signal was detected when samples were not reverse transcribed. Bands at 178 bp (SM-1) and 217 bp (SM-2) were detected with the SM-1/SM-2 primer pair. Molecular weight size markers (MW; 100 bp DNA ladder) are in the right lane. The PCR primers used are given in Table 1.
