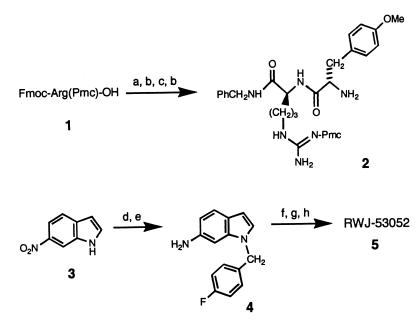
Figure 2.
Synthesis of prototype RWJ-53052 (5). Reaction conditions (yield): a, BnNH2, bis(2-oxo-3-oxazolidinyl)phosphinic chloride, CH2Cl2, 0°C (83%); b, 20% (vol/vol) piperidine in 1,4-dioxane (99%); c, Fmoc-4-methoxyphenylalanine, 1,3-diisopropylcarbodiimide, HOBt, CH2Cl2 (84%); d, 4-fluorobenzyl bromide, Cs2CO3, DMF; e, FeCl3⋅6 H2O, charcoal powder, Me2NNH2, MeOH, reflux (93% for 2 steps); f, 4-nitrophenylchloroformate, i-Pr2NEt, CH2Cl2, −20°C—added 2 and warmed to 23°C (65%); g, 37% aqueous CH2O, pyrrolidine, AcOH (63%); h, CF3CO2H/CH2Cl2 (97%). Abbreviations: Fmoc, 9-fluorenylmethoxycarbonyl; Pmc, 2,2,5,7,8-pentamethylchroman-6-sulfonyl; HOBt, 1-hydroxybenzotriazole; DMF, dimethylformamide.