Abstract
Spontaneous blood pressure (BP) and R-R variability are used frequently as ‘windows’ into cardiovascular control mechanisms. However, the origin of these rhythmic fluctuations is not completely understood. In this study, with ganglion blockade, we evaluated the role of autonomic neural activity versus other ‘non-neural’ factors in the origin of BP and R-R variability in humans. Beat-to-beat BP, R-R interval and respiratory excursions were recorded in ten healthy subjects (aged 30 ± 6 years) before and after ganglion blockade with trimethaphan. The spectral power of these variables was calculated in the very low (0.0078-0.05 Hz), low (0.05-0.15 Hz) and high (0.15-0.35 Hz) frequency ranges. The relationship between systolic BP and R-R variability was examined by cross-spectral analysis. After blockade, R-R variability was virtually abolished at all frequencies; however, respiration and high frequency BP variability remained unchanged. Very low and low frequency BP variability was reduced substantially by 84 and 69 %, respectively, but still persisted. Transfer function gain between systolic BP and R-R interval variability decreased by 92 and 88 % at low and high frequencies, respectively, while the phase changed from negative to positive values at the high frequencies. These data suggest that under supine resting conditions with spontaneous breathing: (1) R-R variability at all measured frequencies is predominantly controlled by autonomic neural activity; (2) BP variability at high frequencies (> 0.15 Hz) is mediated largely, if not exclusively, by mechanical effects of respiration on intrathoracic pressure and/or cardiac filling; (3) BP variability at very low and low frequencies (< 0.15 Hz) is probably mediated by both sympathetic nerve activity and intrinsic vasomotor rhythmicity; and (4) the dynamic relationship between BP and R-R variability as quantified by transfer function analysis is determined predominantly by autonomic neural activity rather than other, non-neural factors.
Rhythmic fluctuations in arterial pressure (BP) and pulse rate have intrigued physiologists since Stephen Hales and Albrecht von Haller first described these phenomena in the eighteenth century (Anrep et al. 1936; Koepchen, 1984). Yet, it was not until the advent of modern computing techniques that quantification of BP and R-R interval variability has been used extensively as a probe for cardiovascular control mechanisms in humans (Malliani et al. 1991). However, despite extensive study, many long-standing controversies remain. For example, it is still not clear whether these rhythmic fluctuations originate centrally from oscillatory neural activities in the medulla and/or in the spinal cord (Levy et al. 1966; Preiss & Polosa, 1974; Koh et al. 1994; Cooley et al. 1998; Montano et al. 2000), or peripherally from baroreflex feedback loops (DeBoer et al. 1987; Cevese et al. 2001). Moreover, although the role of mechanical effects of respiration on oscillations in cardiac output and intrinsic vasomotor rhythmicity (spontaneous vasomotion in peripheral vascular beds) has been recognized by many investigators (Guz et al. 1987; Bouskela & Grampp, 1992; Toska & Eriksen, 1993; Rizzoni et al. 1995), to what extent, in comparison with autonomic neural activity, these factors contribute to the genesis of BP and R-R variability remains unclear. Finally, interactions among cardiovascular variables result in the system being extremely complex. As such, even whether a causal relationship exists between BP and R-R variability is controversial (Baselli et al. 1988; Saul et al. 1991; Taylor & Eckberg, 1996).
In the present study, we blocked both vagal and sympathetic nerve activities simultaneously via ganglion blockade to dissect contributions of autonomic neural activity versus mechanical effects of respiration and intrinsic vasomotor rhythmicity in the genesis of BP and R-R variability in humans. We speculated that (1) if BP variability at high frequencies (> 0.15 Hz) is mediated mainly by the mechanical effects of respiration on intrathoracic pressure and/or cardiac filling, it should remain unchanged by ganglion blockade; (2) if BP variability at low frequencies (< 0.15 Hz) is mediated by both sympathetic nerve activity and intrinsic vasomotor rhythmicity, it should be reduced, but would not be abolished by ganglion blockade; and (3) the dynamic relationship between BP and R-R variability as quantified by transfer function analysis is determined predominantly by autonomic neural activity.
Methods
Subjects
Ten healthy subjects (8 men, 2 women) with a mean age of 30 ± 6 years, height of 173 ± 10 cm, and weight of 69 ± 9 kg participated in this study. No subject smoked, used recreational drugs, or had known medical problems. Subjects were carefully screened with regard to their medical history and a physical examination with 12-lead ECG was performed. The study was performed in accordance with the Declaration of Helsinki and all subjects signed an informed consent form approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and the Presbyterian Hospital of Dallas.
Instrumentation
Heart rate was monitored continuously by ECG. In seven subjects, arterial pressure was measured non-invasively with finger photoplethysmography (Finapres, Ohmeda). In three subjects, pressure was measured simultaneously with a radial artery catheter (18 gauge, Transpac IV, Abbott Critical Care System) and finger photoplethysmography to confirm the reliability of the Finapres during ganglion blockade. The pressure transducer of the intra-arterial catheter was calibrated and zeroed to the mid-axillary line during the experiments. The Finapres transducer was also positioned at heart level. In addition, respiratory excursions were monitored continuously via a piezoelectric transducer during the experiments (Pneumotrace, Morro Bay, CA, USA).
Protocol
All experiments were performed in the morning at least 2 h after a light breakfast in a quiet environmentally controlled laboratory with an ambient temperature of 25 °C. The subjects were asked to refrain from heavy exercise and caffeinated or alcoholic beverages for at least 24 h before the tests. After at least 30 min of supine rest, 6 min of baseline data were collected during spontaneous breathing. This data collection was repeated again after approximately 1 h to test the reproducibility of BP and R-R variability analysis. Then, the subjects performed a Valsalva manoeuvre with an expiratory strain of 30 mmHg for 15 s (Sandroni et al. 1991; Smith et al. 1996). The strain pressure during the Valsalva manoeuvre was monitored by a sphygmomanometer (Tycos, Arden, NC, USA). Typical changes in arterial pressure and heart rate during the Valsalva manoeuvre were observed in all subjects before ganglion blockade (Fig. 1A). After performance of the baseline Valsalva manoeuvre, intravenous infusion of trimethaphan (trimethaphan camsylate, Cambridge Laboratories, UK) was begun at a low dose of 3 mg min−1. Three minutes after the infusion, a Valsalva manoeuvre was performed again to evaluate the heart rate responses to the changes in pressure. The infusion dose was increased incrementally by 1 mg min−1 if the heart rate response during the preceding Valsalva manoeuvre was still present. These procedures were repeated at each level of infusion until the absence of heart rate response was observed (Fig. 1B). The ultimate infusion dose used for ganglion blockade was 6–7 mg min−1 in the present study. The efficacy of ganglion blockade was demonstrated not only by the absence of heart rate response, but also by the absence of BP recovery during phase II or BP overshoot during phase IV of the Valsalva manoeuvre, suggesting the blockade of vasoconstrictor sympathetic nerve activity (Sandroni et al. 1991; Smith et al. 1996).
Figure 1.
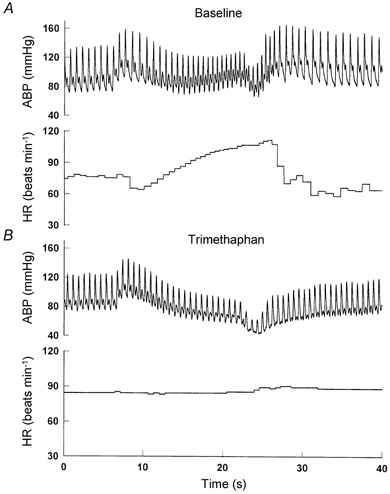
Representative changes in arterial pressure (ABP) and heart rate (HR) during the Valsalva manoeuvre. A, before ganglion blockade; B, after ganglion blockade.
Once complete blockade was achieved, the infusion of trimethaphan continued at this peak dose throughout the experiments. Six minutes of data were collected after the ganglion blockade. To determine whether changes in vascular resistance and/or vasomotor tone associated with the ganglion blockade influence BP and R-R variability directly, low dose phenylephrine was titrated intravenously in three subjects (2 with intra-arterial catheter, 1 with Finapres) to restore the decreased BP to the pre-trimethaphan level. Six minutes of data were collected again after this intervention.
Data analysis
ECG and arterial pressure waveforms were sampled at 1 kHz and digitized at 12 bits with an A/D converter (Das-20, Metrabyte). Respiratory excursions were sampled simultaneously with ECG and BP signals at 10 Hz. Digitized signals were stored on a laboratory computer and processed with a custom-designed program for R wave, and systolic and diastolic pressure detection. Beat-to-beat R-R interval, systolic and diastolic pressure, and respiratory excursions were linearly interpolated and then resampled at 2 Hz for spectral analysis. The time series of R-R interval, and systolic and diastolic pressure were first detrended with third-order polynomial fitting and then subdivided into 256 point segments with 50 % overlap for spectral estimation. This process resulted in five segments of data over the 6 min period recordings. Fast Fourier transforms were then implemented with each Hanning-windowed data segment and then averaged to calculate auto-spectra, cross-spectra, coherence and transfer functions. The spectral resolution for these estimates is ≈0.0078 Hz.
The spectral power of R-R interval, systolic BP (SBP) and diastolic BP (DBP) was calculated in the very low (0.0078-0.05 Hz), low (0.05-0.15 Hz) and high (0.15-0.35 Hz) frequency ranges by integrating the corresponding auto-spectra (Koh et al. 1994; Taylor & Eckberg, 1996; Cooke et al. 1999; Iwasaki et al. 2000). Moreover, respiratory frequency was identified from the peak position of the auto-spectrum of respiratory excursions, and the spectral power was calculated in the high frequency range to reflect relative changes in lung volume (Saul et al. 1991). For the cross-spectral analysis, mean values of transfer function gain, phase and coherence were calculated in the low and high frequency ranges. In this study, a lower limit of 0.05 Hz was selected for calculation of both low frequency spectral power and the transfer function gain and phase. This selection was based on the consideration that coherence function in general was too low below 0.05 Hz, and may compromise transfer function estimates at lower frequencies (Saul et al. 1991; Iwasaki et al. 2000).
Statistics
Mean values and standard deviations of R-R interval, SBP and DBP were calculated first over the 6 min data segments for each individual subject, and then group averaged. Student's paired t tests were performed to test the reproducibility of BP and R-R variability and to compare the variables before and after ganglion blockade. The normality of data was confirmed by the Kolmogorov test (SigmaStat, SPSS Inc.). Logarithmic transformation was performed if the spectral power estimates were not normally distributed. However, this data transformation did not influence the outcome of the statistical analysis before and after ganglion blockade. Data are expressed as means ± s.e.m. The significance level was set to P < 0.05.
Results
Differences of 8–12 mmHg in the steady-state SBP and 3–4 mmHg in DBP were observed between the arterial catheter and finger photoplethysmography methods. However, the reductions in SBP associated with the ganglion blockade measured by the two methods were similar (13 % in arterial catheter, 11 % in Finapres, n = 3). Moreover, a significant linear relationship for the spectral power estimates of SBP and DBP at all frequencies was observed between the two methods (r2 = 0.96, slope = 1.28, intercept = 0.12 mmHg2). These data, consistent with previous findings (Parati et al. 1989; Omboni et al. 1993), confirm the reliability of using finger photoplethysmography for measurements of changes in BP under the conditions of the present study. Furthermore, no significant difference in spectral power estimates of BP and R-R variability was found between the repeated baseline measurements, confirming the reproducibility of short-term BP and R-R variability analysis under well-controlled experimental conditions (Dimier-David et al. 1994).
After ganglion blockade, R-R interval decreased by 29 % and SBP decreased by 13 % (Table 1). No significant changes were observed in DBP, respiratory frequency or respiratory excursion power (Table 1). These changes in steady-state haemodynamics were associated with an overall reduction of R-R and BP variability in the time domain reflected by the reductions in the signal standard deviation (Table 1).
Table 1.
Steady-state haemodynamics before and after ganglion blockade
| Baseline | Blockade | P | |
|---|---|---|---|
| R–R (ms) | 998 ± 52 | 712 ± 23 | <0.001 |
| RRSD (ms) | 49 ± 5 | 5 ± 1 | <0.001 |
| SBP (mmHg) | 126 ± 3 | 109 ± 5 | 0.004 |
| SBPSD (mmHg) | 4.4 ± 0.5 | 2.4 ± 0.2 | 0.001 |
| DBP (mmHg) | 67 ± 2 | 65 ± 3 | 0.558 |
| DBPSD (mmHg) | 2.7 ± 0.3 | 1.5 ± 0.2 | 0.001 |
| Respiratory frequency (Hz) | 0.28 ± 0.02 | 0.27 ± 0.02 | 0.345 |
| Respiratory excursion power (units) | 7 ± 2 | 8 ± 3 | 0.312 |
n = 10, values are presented as means ± s.e.m. R–R, R–R interval; RRSD, standard deviation of R–R interval calculated from 6 min data segments; SBP, systolic pressure; SBPSD, standard deviation of SBP calculated from 6 min data segments; DBP, diastolic pressure; DBPSD standard deviation of DBP calculated from 6 min data segments.
After ganglion blockade, R-R variability was virtually abolished at all frequencies (Table 2, Figs 2–4). However, small fluctuations synchronized with respiration still persisted (Table 2, Fig. 2 and Fig. 3). SBP variability decreased substantially by 69 %, and DBP variability decreased by 78 %, at low frequencies; similar reductions in SBP variability (84 %) and DBP variability (69 %) were also observed in the very low frequency range, although the absolute magnitude of these estimates is less certain due to the relatively small number of observations at the lower end of this range (Table 2, Fig. 2 and Fig. 3). In contrast, no change in BP variability at high frequencies, which was small relative to those at the very low and low frequencies, was observed (Table 2, Figs 2–4).
Table 2.
Spectral analysis of R–R interval and arterial pressure variability before and after ganglion blockade
| Very low frequency | Low frequency | High frequency | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Blockade | P | Baseline | Blockade | P | Baseline | Blockade | P | |
| R–R (ms2) | 740 ± 162 | 5 ± 1 | 0.001 | 718 ± 130 | 1 ± 0 | <0.001 | 367 ± 87 | 4 ± 1 | 0.003 |
| SBP (mmHg2) | 11.4 ± 2.6 | 1.8 ± 0.4 | 0.004 | 4.5 ± 1.0 | 1.4 ± 0.7 | 0.003 | 1.1 ± 0.3 | 1.2 ± 0.3 | 0.729 |
| DBP (mmHg2) | 4.2 ± 1.0 | 1.3 ± 0.3 | 0.006 | 2.3 ± 0.5 | 0.5 ± 0.2 | 0.008 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.343 |
n = 10, values are presented as means ± s.e.m. Very low frequency range, 0.0078–0.05 Hz; low frequency range, 0.05–0.15 Hz; high frequency range, 0.15–0.35 Hz.
Figure 2.
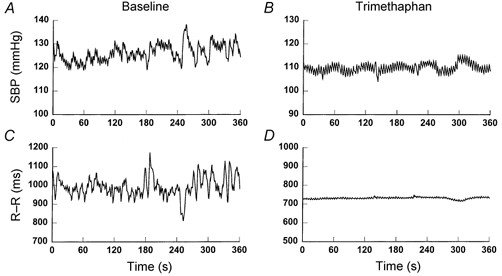
Representative time series of systolic pressure (SBP) and R-R interval before (A and C) and after (B and D) ganglion blockade.
Figure 4.
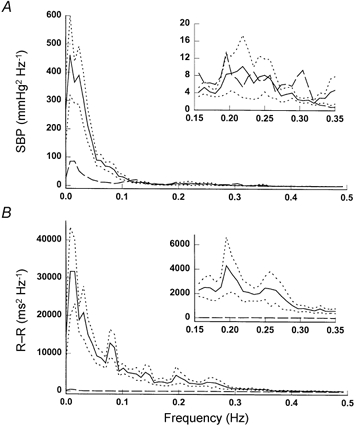
Group averaged spectra of SBP (A) and R-R interval (B) variability before (continuous lines) and after (dashed lines) ganglion blockade. Dotted lines, s.e.m. Note that the plots in the insets have ‘zoomed’ scales at the frequencies from 0.15 to 0.35 Hz for the SBP spectrum in A and for the R-R spectrum in B.
Figure 3.
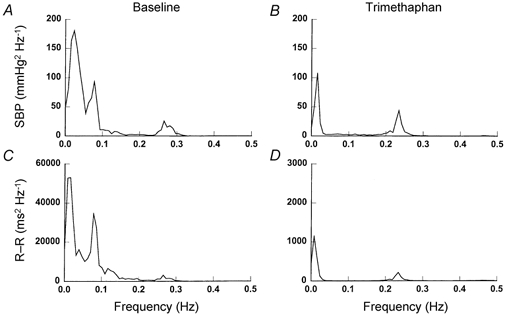
Representative spectra of SBP and R-R interval variability before (A and C) and after (B and D) ganglion blockade. Data are from the same subject as in Fig. 2. Note that the y-axis scale of D is 1/20 of that of C after ganglion blockade.
Transfer function gain between SBP and R-R variability decreased after ganglion blockade by 92 and 88 % at the low and high frequencies, respectively, while the phase changed from negative to positive values at the high frequencies (Table 3, Fig. 5). Interestingly, the coherence function remained unchanged at the low frequencies, and even increased significantly at the high frequencies after ganglion blockade (Table 3, Fig. 5).
Table 3.
Transfer function analysis of systolic pressure and R–R variability before and after ganglion blockade
| Low frequency | High frequency | |||||
|---|---|---|---|---|---|---|
| Baseline | Blockade | P | Baseline | Blockade | P | |
| Gain (ms mmHg−1) | 11.3 ± 1.4 | 0.9 ± 0.1 | <0.001 | 14.3 ± 2.0 | 1.7 ± 0.3 | <0.001 |
| Phase (rad) | -1.0 ± 0.1 | -0.2 ± 0.3 | 0.08 | -0.4 ± 0.1 | 0.6 ± 0.1 | 0.001 |
| Coherence | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.28 | 0.5 ± 0.1 | 0.8 ± 0.1 | 0.002 |
n = 10, values are presented as means ± s.e.m. Low frequency range, 0.05–0.15 Hz; high frequency range, 0.15–0.35 Hz.
Figure 5.
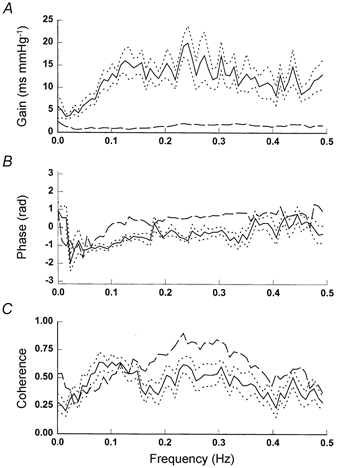
Group averaged transfer function gain (A), phase (B) and coherence (C) before (continuous lines) and after (dashed lines) ganglion blockade. Dotted lines, s.e.m.
Finally, in three subjects, restoring SBP to the baseline level (prior to ganglion blockade) with phenylephrine did not affect the observed changes in BP and R-R variability, suggesting that these changes were not likely to be caused by the reduction of baseline BP associated with the ganglion blockade (Fig. 6 and Fig. 7).
Figure 6.
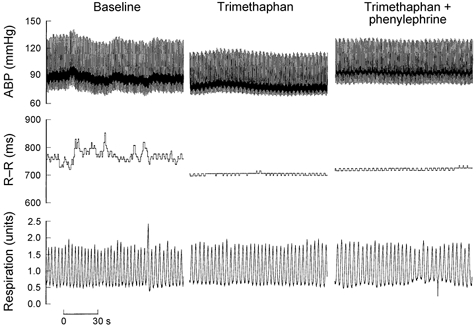
Direct recordings of ABP, R-R interval and respiration at baseline (left), and during trimethaphan (middle) and trimethaphan plus phenylephrine (right) infusion.
Figure 7.
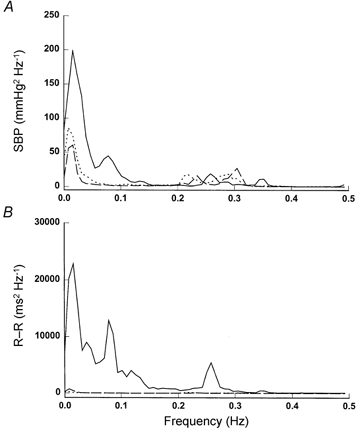
Group averaged spectra of SBP (A) and R-R interval (B) variability from three subjects at baseline (continuous lines), and during trimethaphan (dashed lines) and trimethaphan plus phenylephrine (dotted lines) infusion.
Discussion
Our study advances the understanding of human physiology in the following ways. (1) We demonstrated for the first time that, under supine resting conditions, BP variability at low frequencies (< 0.15 Hz) was substantially reduced, but still persisted after ganglion blockade. These data suggest that sympathetic nerve activity is a critical determinant of this rhythm; however, intrinsic vasomotor rhythmicity is also likely to play a role. (2) BP variability at high frequencies (> 0.15 Hz) remained unchanged, even though R-R variability was virtually abolished by ganglion blockade, providing further evidence that high frequency BP variability is determined largely, if not exclusively, by mechanical effects of respiration on intrathoracic pressure and/or cardiac filling. (3) Transfer function gain between BP and R-R variability decreased substantially at both low and high frequencies after ganglion blockade, while the phase changed from negative to positive values at the high frequencies. These data show clearly that the dynamic relationship between BP and R-R variability is determined predominantly by autonomic neural activity rather than by other non-neural factors.
Low frequency BP variability
Several lines of evidence suggest that sympathetic nerve activity is a critical determinant of low frequency BP variability. First, spontaneous fluctuations of muscle sympathetic nerve activity (MSNA) at low frequencies have been observed similar to those in BP variability (Pagani et al. 1997; Furlan et al. 2000). Moreover, low frequency BP variability increased simultaneously with MSNA during head-up tilt (Cooke et al. 1999), and was substantially reduced by peripheral α-adrenergic blockade (Cevese et al. 2001). Second, with local perfusion pressure maintained constant, blood flow in mechanically isolated vascular beds still fluctuated at low frequencies similar to those observed in systemic BP (Cevese et al. 1995). In addition, low frequency BP variability persisted in dogs when cardiac output was clamped at a constant level (O'Leary & Woodbury, 1996). These findings suggest that low frequency BP variability is mediated by changes in peripheral vascular resistance, which in turn are determined by the changes in sympathetic nerve activity.
The present study extends these previous observations by showing that BP variability at both low (0.05-0.15 Hz) and very low frequencies (0.0078-0.05 Hz) was markedly reduced after ganglion blockade. These data, to the best of our knowledge, document for the first time the obligatory role of sympathetic nerve activity in the genesis of low and very low frequency BP variability in humans. However, the absolute magnitude of the reduction in very low frequency BP variability should be interpreted with caution because of the relatively few samples available at the lower end of this range.
A more novel observation of the present study is that a considerable amount of low frequency BP variability persisted nevertheless after ganglion blockade. We interpret this observation as suggesting a contribution of intrinsic vasomotor rhythmicity to the origin of low frequency BP variability in humans. Intrinsic vasomotor rhythmicity at both very low and low frequencies has been observed ubiquitously in peripheral vascular beds both in vivo and in vitro, and has been suggested by others to play an important role in the origin of BP variability (Bouskela & Grampp, 1992; Rizzoni et al. 1995). Since intrinsic vasomotor rhythmicity is more likely to be controlled by local mechanisms than by autonomic neural activity (Bouskela & Grampp, 1992; Rizzoni et al. 1995), we speculate that their contributions to BP variability were still present after ganglion blockade. However, the specific mechanism(s) underlying this process is unknown (Gustafsson, 1993). Moreover, the relative contribution of neural activity versus intrinsic vasomotor activity in the genesis of BP variability may vary among individuals and under different experimental conditions. Thus, changes in low frequency BP variability should be interpreted judiciously regarding its use to reflect changes in sympathetic nerve activity (Parati et al. 1995).
High frequency BP variability
In the present study, we observed that BP variability at high frequencies remained unchanged even though R-R variability was abolished by ganglion blockade. These data, consistent with the findings in patients with heart transplantation (Macor et al. 1994), and in animals with ganglion blockade (Cerutti et al. 1994), provide further evidence that, under supine resting conditions during spontaneous breathing, BP variability at high respiratory frequencies is mediated to a large extent by mechanical effects of respiration on intrathoracic pressure and/or cardiac filling, and is less influenced by ‘feed-forward’ effects of changes in R-R interval on BP variability and/or changes in peripheral vascular resistance (Dornhorst et al. 1952; Rosenbaum & Race, 1968; Akselrod et al. 1985).
The interpretation of experimental observations regarding the effects of R-R variability on BP variability is controversial (Saul et al. 1991; Toska & Eriksen, 1993; Taylor & Eckberg, 1996). In some studies, R-R variability was abolished either by cardiac autonomic receptor blockade (Saul et al. 1991; Toska & Eriksen, 1993), or by atrial pacing (Akselrod et al. 1985; Taylor & Eckberg, 1996). Under these circumstances, BP variability at respiratory frequencies has been reported to be either enhanced (Toska & Eriksen, 1993), or attenuated (Akselrod et al. 1985; Taylor & Eckberg, 1996). Further analysis also suggests that these effects may be both frequency and posture dependent in humans (Saul et al. 1991; Taylor & Eckberg, 1996). These findings appear inconsistent with the absence of changes in high frequency BP variability observed in the present study. We speculate that a fundamental difference, which might lead to this discrepancy, is in the methods used for autonomic blockade. In the present study, trimethaphan infusion blocked both vagal and sympathetic nerve activity simultaneously to the heart and peripheral vascular bed. However, vascular sympathetic nerve activity was not blocked in previous studies with either cardiac autonomic receptor blockade or atrial pacing (Saul et al. 1991; Toska & Eriksen, 1993; Taylor & Eckberg, 1996). Moreover, cardiac autonomic receptor blockade may modulate sympathetic nerve activity centrally (Montano et al. 1998), and sympathetic nerve activity coupling to peripheral vascular resistance directly (Jacobsen et al. 1992). Therefore, it is possible that even though R-R variability was eliminated similarly in these protocols, the presence of sympathetic nerve activity to peripheral vascular beds with either cardiac autonomic receptor blockade or pacing may affect BP variability differently from that of the present study.
R-R variability
Consistent with previous findings, R-R variability was virtually abolished at all frequencies after ganglion blockade (Casadei et al. 1996; El-Omar et al. 2001). However, small R-R fluctuations synchronized with respiration were still present. We cannot exclude the possibility that the small R-R fluctuations observed in the present study may signify sinus node responses to extremely small but persistently unblocked vagal activity. However, these data confirm that R-R variability is mediated overwhelmingly by autonomic neural activity (Pomeranz et al. 1985); thus any contribution of sinus node stretching associated with respiration to the genesis of R-R variability, if present, must be very small (Bernardi et al. 1989; Casadei et al. 1996; El-Omar et al. 2001).
Implications of cross-spectral analysis
Transfer function analysis of BP and R-R variability has been used extensively to evaluate baroreflex function in humans (DeBoer et al. 1987; Cooke et al. 1999; Iwasaki et al. 2000; Cevese et al. 2001). The fundamental concept of this method was pioneered by DeBoer and his colleagues, assuming that R-R variability originates peripherally from changes in BP mediated via the baroreflex (DeBoer et al. 1987). This hypothesis has been tested elegantly in humans (DeBoer et al. 1987; Cevese et al. 2001). For example, at low frequencies, removal of BP variability with α-adrenergic blockade abolished R-R variability, suggesting that low frequency R-R variability is induced by the changes in BP via the baroreflex (Cevese et al. 2001). At high respiratory frequencies, although it has been difficult to dissect central origins from peripheral baroreflex mechanisms in the genesis of BP and R-R variability in humans, mathematical model simulation suggests that R-R variability at respiratory frequencies originates primarily from BP variability via the baroreflex (DeBoer et al. 1987). These data would suggest that R-R variability at both low and high frequencies is generated by BP variability via the baroreflex.
However, other findings, mostly in patients with severe diseases or injuries, and in animals, are in conflict with the above baroreflex hypothesis (Preiss & Polosa, 1974; Cooley et al. 1998; Montano et al. 2000). For example, it has been shown that low frequency BP and R-R variability may originate centrally from oscillatory neural activity in the medulla and/or in the spinal cord (Fernandez de Molina & Perl, 1965; Preiss & Polosa, 1974; Koh et al. 1994; Cooley et al. 1998; Montano et al. 2000). Moreover, at high frequencies, phasic changes in respiratory neural activity may modulate motoneuronal activity of the cardiovascular centre and cause respiratory fluctuations in efferent sympathetic and/or vagal nerve activity, and hence R-R variability (Eckberg et al. 1980). Consequently, these data would suggest that, in contrast to the baroreflex mechanism, BP and R-R variability may occur coincidentally rather than causally via a central mechanism.
The data of transfer function analysis in the present study are consistent with previous findings in healthy subjects (Cooke et al. 1999; Iwasaki et al. 2000; Cevese et al. 2001). Before ganglion blockade, transfer function gain showed typical properties of a band-pass filter associated with a gradual decrease in negative phase with increases in frequency. After ganglion blockade, transfer function gain decreased substantially at both low and high frequencies and phase changed from negative to positive values at the high frequencies. These data demonstrated convincingly the obligatory role of autonomic neural control of the dynamic relationship between BP and R-R variability at all frequencies in humans.
However, since ganglion blockade blocked both sympathetic and parasympathetic nerve activity regardless of where and how it originated, the changes in transfer function gain and phase after ganglion blockade cannot be taken as proof either for or against the central or the baroreflex mechanism in the genesis of BP and R-R variability at any frequency measured in the present study. This issue is highlighted further by the fact that the coherence function remained unchanged at low frequencies and even increased significantly at high frequencies after ganglion blockade. These data, consistent with the findings in animals with sinoaortic denervation (Mancia et al. 1999), suggest that a high coherence between BP and R-R variability at a given frequency does not necessarily imply causality, and emphasizes the limitations of using this index by itself to indicate statistical reliability of transfer function estimates.
In the present study, we have been cautious not to calculate ‘baroreflex latency’ based on the estimated phase (DeBoer et al. 1987; Taylor & Eckberg, 1996; Cevese et al. 2001). As a common practice, identification of a negative phase between BP and R-R variability has been interpreted to indicate that changes in BP lead the changes in R-R interval (DeBoer et al. 1987; Cevese et al. 2001). In addition, assuming that baroreflex control of heart rate could be modelled by a static function with a pure time delay, the estimated phase has been used to calculate the baroreflex latency (Taylor & Eckberg, 1996; Cevese et al. 2001). However, studies both in animals and in humans showed clearly that baroreflex control of heart rate possesses higher order dynamics (≥ 2) than that of a simply static function with a pure time delay (Mokrane, 1995; Kawada et al. 1996; Zhang et al. 2001). Thus, the negative phase observed before, and the phase change after ganglion blockade observed in the present study should not be interpreted to reflect any changes in baroreflex latency and indicate any causal relationship between BP and R-R variability. Rather, these data emphasize that the temporal relationship between high frequency BP and R-R variability, both of which are directly and/or indirectly generated by respiration, is modulated importantly by autonomic neural activity.
Study limitations
Although it has been shown that ganglion blocking agents have no direct effects on cardiac muscle (Lee & Shideman, 1958; Aviado, 1960), vasodilatation induced by ganglion blockade of sympathetic nerve activity has been reported (Aviado, 1960). Systemic BP may either remain unchanged or fall, depending on how important each individual's vascular resistance and cardiac output are for BP control (Aviado, 1960). In the present study, after ganglion blockade, HR increased, SBP decreased and DBP remained unchanged. These data, consistent with previous findings, suggest a reduction of peripheral vascular resistance and/or vasomotor tone along with the unmasking of intrinsic HR after ganglion blockade (Aviado, 1960; Jose & Taylor, 1969). Since changes in vascular resistance and/or vasomotor tone may affect not only cardiovascular coupling (Nichols & O'Rourke, 1990), but also intrinsic vasomotor rhythmicity, and hence BP variability (Julien et al. 1993; Rizzoni et al. 1995), phenylephrine was used to restore the reduced SBP to pre-trimethaphan levels in three subjects in the present study. This intervention had no effect on the observed changes in BP and R-R variability. Therefore, we conclude that with the degree of BP reduction in the present study, alterations in vascular resistance and/or vasomotor tone associated with the ganglion blockade are unlikely to play a major role in mediating the observed changes in BP variability. However, the small number of subjects exposed to this intervention must be acknowledged.
In addition, in the present study, we did not directly measure sympathetic nerve activity or mechanical effects of respiration on intrathoracic pressure and/or cardiac filling. Thus, the interpretation of the data is speculative and limited as to the specific mechanisms underlying the observed changes in BP and R-R variability. However, other investigators have confirmed that MSNA is completely abolished by infusion of trimethaphan in humans at doses similar to those used herein (Shannon et al. 1998). Furthermore, failure of BP to recover during phase II and the absence of BP overshoot during phase IV of the Valsalva manoeuvre also suggests blockade of sympathetic nerve activity (Sandroni et al. 1991; Smith et al. 1996). Finally, mechanical effects of respiration on intrathoracic pressure and/or cardiac filling have been reported by many other investigators (Guz et al. 1987; Toska & Eriksen, 1993). Thus, BP may be affected directly by the changes in intrathoracic pressure and/or indirectly by effects of changes in cardiac filling on cardiac output via Starling's law (Toska & Eriksen, 1993; Levine et al. 1996). We assume that, since respiration did not change with ganglion blockade, the mechanical effects of respiration on BP and R-R variability remained unchanged in the present study.
In summary, with ganglion blockade, we have demonstrated a critical role of sympathetic nerve activity in the origin of low frequency BP variability. However, persistent BP variability after ganglion blockade also reveals the contribution of intrinsic vasomotor rhythmicity in the genesis of low frequency BP variability in humans. Moreover, we found that high frequency BP variability remained unchanged even though R-R variability was virtually abolished after ganglion blockade. This observation provides further evidence that high frequency BP variability may be mediated largely, if not exclusively, by mechanical effects of respiration on intrathoracic pressure and/or cardiac filling. Finally, the substantially reduced transfer function gain and changes in phase after ganglion blockade reveal an important role of autonomic neural activity, as opposed to other, non-neural factors in the determination of the dynamic relationship between BP and R-R variability.
Acknowledgments
This study was supported by a grant from The American Heart Association Texas Affiliate grant-in-aid 98BG058 and NIH Neurolab grant HL 53206-03.
References
- Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectral analysis. American Journal of Physiology. 1985;249:H867–875. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- Anrep GV, Pascual W, Rossler R. Respiratory variations of the heart rate, I – the reflex mechanism of respiratory arrhythmia. Proceedings of the Royal Society of Edinburgh B. 1936;119B:191–217. [Google Scholar]
- Aviado DM. Hemodynamic effects of ganglion blocking drugs. Circulation Research. 1960;8:304–314. [Google Scholar]
- Baselli G, Cerutti S, Civardi S, Malliani A, Pagani M. Cardiovascular variability signals: towards the identification of a closed-loop model of the neural control mechanisms. IEEE Transactions on Biomedical Engineering. 1988;35:1033–1046. doi: 10.1109/10.8688. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Keller F, Sanders M, Reddy PS, Griffith B, Meno F, Pinsky MR. Respiratory sinus arrhythmia in the denervated human heart. Journal of Applied Physiology. 1989;67:1447–1455. doi: 10.1152/jappl.1989.67.4.1447. [DOI] [PubMed] [Google Scholar]
- Bouskela E, Grampp W. Spontaneous vasomotion in hamster cheek pouch arterioles in varying experimental conditions. American Journal of Physiology. 1992;262:H478–485. doi: 10.1152/ajpheart.1992.262.2.H478. [DOI] [PubMed] [Google Scholar]
- Casadei B, Moon J, Johnston J, CaiaAZZ A, Sleight P. Is respiratory sinus arrhythmia a good index of cardiac vagal tone in exercise? Journal of Applied Physiology. 1996;81:556–564. doi: 10.1152/jappl.1996.81.2.556. [DOI] [PubMed] [Google Scholar]
- Cevese A, Gulli G, Polati E, Gottin L, Grasso R. Baroreflex and oscillation of heart period at 0. 1 Hz studied by α-blockade and cross-spectral analysis in healthy humans. Journal of Physiology. 2001;531:235–244. doi: 10.1111/j.1469-7793.2001.0235j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevese A, Grasso R, Poltronieri R, Schena F. Vascular resistance and arterial pressure low-frequency oscillations in the anesthetized dog. American Journal of Physiology. 1995;268:H7–16. doi: 10.1152/ajpheart.1995.268.1.H7. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Human responses to upright tilt: a window on central autonomic integration. Journal of Physiology. 1999;517:617–628. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley RL, Montano N, Cogliati C, Van De Borne P, Richenbacher W, Oren R, Somers VK. Evidence for a central origin of the low-frequency oscillation in RR-interval variability. Circulation. 1998;98:556–561. doi: 10.1161/01.cir.98.6.556. [DOI] [PubMed] [Google Scholar]
- DeBoer RW, Karemaker JM, Strackee J. Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. American Journal of Physiology. 1987;253:H680–689. doi: 10.1152/ajpheart.1987.253.3.H680. [DOI] [PubMed] [Google Scholar]
- Dimier-David L, Billon N, Costagliola D, Jaillon P, Funck-Brentano C. Reproducibility of non-invasive measurement and of short-term variability of blood pressure and heart rate in healthy volunteers. British Journal of Clinical Pharmacology. 1994;38:109–115. doi: 10.1111/j.1365-2125.1994.tb04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornhorst AC, Howard P, Leathart GL. Respiratory variations in blood pressure. Circulation. 1952;6:553–558. doi: 10.1161/01.cir.6.4.553. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Kifle YT, Roberts VL. Phase relationship between normal human respiration and baroreflex responsiveness. Journal of Physiology. 1980;304:489–502. doi: 10.1113/jphysiol.1980.sp013338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Omar M, Kardos A, Casadei B. Mechanisms of respiratory sinus arrhythmia in patients with mild heart failure. American Journal of Physiology – Heart and Circulatory Physiology. 2001;280:H125–131. doi: 10.1152/ajpheart.2001.280.1.H125. [DOI] [PubMed] [Google Scholar]
- FernandeZ de Molina AF, Perl ER. Sympathetic activity and the systemic circulation in the spinal cat. Journal of Physiology. 1965;181:82–102. doi: 10.1113/jphysiol.1965.sp007747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan R, Porta A, Costa F, Tank J, Baker L, Schiavi R, Robertson D, Malliani A, Mosqueda-Garcia R. Oscillatory patterns in sympathetic neural discharge and cardiovascular variables during orthostatic stimulus. Circulation. 2000;101:886–892. doi: 10.1161/01.cir.101.8.886. [DOI] [PubMed] [Google Scholar]
- Gustafsson H. Vasomotion and underlying mechanisms in small arteries. An in vitro study of rat blood vessels. Acta Physiologica Scandinavica Supplementum. 1993;614:1–44. [PubMed] [Google Scholar]
- Guz A, Innes JA, Murphy K. Respiratory modulation of left ventricular stroke volume in man measured using pulsed Doppler ultrasound. Journal of Physiology. 1987;393:499–512. doi: 10.1113/jphysiol.1987.sp016836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki KI, Zhang R, Zuckerman JH, Pawelczyk JA, Levine BD. Effect of head-down-tilt bed rest and hypovolemia on dynamic regulation of heart rate and blood pressure. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2000;279:R2189–2199. doi: 10.1152/ajpregu.2000.279.6.R2189. [DOI] [PubMed] [Google Scholar]
- Jacobsen TN, Converse RL, Jr, Victor RG. Contrasting effects of propranolol on sympathetic nerve activity and vascular resistance during orthostatic stress. Circulation. 1992;85:1072–1076. doi: 10.1161/01.cir.85.3.1072. [DOI] [PubMed] [Google Scholar]
- Jose AD, Taylor RR. Autonomic blockade by propranolol and atropine to study intrinsic myocardial function in man. Journal of Clinical Investigation. 1969;48:2019–2031. doi: 10.1172/JCI106167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien C, Zhang ZQ, Barres C. Role of vasoconstrictor tone in arterial pressure lability after chronic sympathectomy and sinoaortic denervation in rats. Journal of the Autonomic Nervous System. 1993;42:1–10. doi: 10.1016/0165-1838(93)90336-s. [DOI] [PubMed] [Google Scholar]
- Kawada T, Ikeda Y, Sugimachi M, Shishido T, Kawaguchi O, Yamazaki T, Alexander J, Jr, Sunagawa K. Bidirectional augmentation of heart rate regulation by autonomic nervous system in rabbits. American Journal of Physiology. 1996;271:H288–295. doi: 10.1152/ajpheart.1996.271.1.H288. [DOI] [PubMed] [Google Scholar]
- Koepchen HP. History of studies and concepts of blood pressure waves. In: Miyakawa K, Koepchen HP, Polosa C, editors. Mechanisms of Blood Pressure Waves. Berlin: Springer-Verlag; 1984. pp. 3–23. [Google Scholar]
- Koh J, Brown TE, Beightol LA, Ha CY, Eckberg DL. Human autonomic rhythms: vagal cardiac mechanisms in tetraplegic subjects. Journal of Physiology. 1994;474:483–495. doi: 10.1113/jphysiol.1994.sp020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Shideman FE. Inotropic action of hexamethonium. Circulation Research. 1958;6:66–71. doi: 10.1161/01.res.6.1.66. [DOI] [PubMed] [Google Scholar]
- Levine BD, Zhang R, Zuckerman JH, Morrow M. The effect of cardiac mechanics on heart rate variability. Circulation. 1996;94:1, 368. [Google Scholar]
- Levy MN, Degeest H, Zieske H. Effects of respiratory center activity on the heart. Circulation Research. 1966;18:67–78. doi: 10.1161/01.res.18.1.67. [DOI] [PubMed] [Google Scholar]
- Macor F, Fagard R, Vanhaecke J, Amery A. Respiratory-related blood pressure variability in patients after heart transplantation. Journal of Applied Physiology. 1994;76:1961–1962. doi: 10.1152/jappl.1994.76.5.1961. [DOI] [PubMed] [Google Scholar]
- Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- Mancia G, Parati G, Castiglioni P, Di Rienzo M. Effect of sinoaortic denervation on frequency-domain estimates of baroreflex sensitivity in conscious cats. American Journal of Physiology. 1999;276:H1987–1993. doi: 10.1152/ajpheart.1999.276.6.H1987. [DOI] [PubMed] [Google Scholar]
- Mokrane A, Leblanc AR, Nadeau R. Transfer function analysis of vagal control of heart rate during synchronized vagal stimulation. American Journal of Physiology. 1995;269:H1931–1940. doi: 10.1152/ajpheart.1995.269.6.H1931. [DOI] [PubMed] [Google Scholar]
- Montano N, Cogliati C, Da Silva VJ, Gnecchi-Ruscone T, Massimini M, Porta A, Malliani A. Effects of spinal section and of positive-feedback excitatory reflex on sympathetic and heart rate variability. Hypertension. 2000;36:1029–1034. doi: 10.1161/01.hyp.36.6.1029. [DOI] [PubMed] [Google Scholar]
- Montano N, Cogliati C, Porta A, Pagani M, Malliani A, NarkiewicZ K, Abboud FM, Birkett C, Somers VK. Central vagotonic effects of atropine modulate spectral oscillations of sympathetic nerve activity. Circulation. 1998;98:1394–1399. doi: 10.1161/01.cir.98.14.1394. [DOI] [PubMed] [Google Scholar]
- Nichols WW, O'Rourke MF. Coupling of the left ventricle with the systemic arterial circulation. In: Nichols WW, O'Rourke MF, editors. McDonald's Blood Flow in Arteries: Theoretic, Experimental and Clinical Principles. Philadelphia: Lea & Febiger; 1990. pp. 343–359. [Google Scholar]
- O'Leary DS, Woodbury DJ. Role of cardiac output in mediating arterial blood pressure oscillations. American Journal of Physiology. 1996;271:R641–646. doi: 10.1152/ajpregu.1996.271.3.R641. [DOI] [PubMed] [Google Scholar]
- Omboni S, Parati G, Frattola A, Mutti E, Di Rienzo M, Castiglioni P, Mancia G. Spectral and sequence analysis of finger blood pressure variability. Comparison with analysis of intra-arterial recordings. Hypertension. 1993;22:26–33. doi: 10.1161/01.hyp.22.1.26. [DOI] [PubMed] [Google Scholar]
- Pagani M, Montano N, Porta A, Malliani A, Abboud FM, Birkett C, Somers VK. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95:1441–1448. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension. 1989;13:647–655. doi: 10.1161/01.hyp.13.6.647. [DOI] [PubMed] [Google Scholar]
- Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension. 1995;25:1276–1286. doi: 10.1161/01.hyp.25.6.1276. [DOI] [PubMed] [Google Scholar]
- Pomeranz B, Macaulay RJ, Caudill MA, KutZ I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ, Benson H. Assessment of autonomic function in humans by heart rate spectral analysis. American Journal of Physiology. 1985;248:H151–153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- Preiss G, Polosa C. Patterns of sympathetic neuron activity associated with Mayer waves. American Journal of Physiology. 1974;226:724–730. doi: 10.1152/ajplegacy.1974.226.3.724. [DOI] [PubMed] [Google Scholar]
- Rizzoni D, Castellano M, Porteri E, Bettoni G, Muiesan P, Muiesan ML, Giulini SM, Cinelli A, Salvetti M, Agabiti Rosei E. Arterial spontaneous rhythmic contractile activity in humans and rats: spectral analysis and regulatory mechanisms. Journal of Hypertension. 1995;13:1043–1052. doi: 10.1097/00004872-199509000-00016. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Race D. Frequency-response characteristics of vascular resistance vessels. American Journal of Physiology. 1968;215:1397–1402. doi: 10.1152/ajplegacy.1968.215.6.1397. [DOI] [PubMed] [Google Scholar]
- Sandroni P, Benarroch EE, Low PA. Pharmacological dissection of components of the Valsalva maneuver in adrenergic failure. Journal of Applied Physiology. 1991;71:1563–1567. doi: 10.1152/jappl.1991.71.4.1563. [DOI] [PubMed] [Google Scholar]
- Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: unique insights into cardiovascular regulation. American Journal of Physiology. 1991;261:H1231–1245. doi: 10.1152/ajpheart.1991.261.4.H1231. [DOI] [PubMed] [Google Scholar]
- Shannon JR, Jordan J, Black BK, Costa F, Robertson D. Uncoupling of the baroreflex by N(N)-cholinergic blockade in dissecting the components of cardiovascular regulation. Hypertension. 1998;32:101–107. doi: 10.1161/01.hyp.32.1.101. [DOI] [PubMed] [Google Scholar]
- Smith ML, Beightol LA, Fritsch-Yelle JM, Ellenbogen KA, Porter TR, Eckberg DL. Valsalva's maneuver revisited: a quantitative method yielding insights into human autonomic control. American Journal of Physiology. 1996;271:H1240–1249. doi: 10.1152/ajpheart.1996.271.3.H1240. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Eckberg DL. Fundamental relations between short-term RR interval and arterial pressure oscillations in humans. Circulation. 1996;93:1527–1532. doi: 10.1161/01.cir.93.8.1527. [DOI] [PubMed] [Google Scholar]
- Toska K, Eriksen M. Respiration-synchronous fluctuations in stroke volume, heart rate and arterial pressure in humans. Journal of Physiology. 1993;472:501–512. doi: 10.1113/jphysiol.1993.sp019958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Behbehani K, Crandall CG, Zuckerman JH, Levine BD. Dynamic regulation of heart rate during acute hypotension: new insight into baroreflex function. American Journal of Physiology – Heart and Circulatory Physiology. 2001;280:H407–419. doi: 10.1152/ajpheart.2001.280.1.H407. [DOI] [PubMed] [Google Scholar]


