Abstract
The aim of this study was to determine in the near-term ovine fetus the role of adenosine in the basal regulation of cerebral blood flow and in the increases in cerebral blood flow in response to acute hypoxic insult. We measured cerebral blood flow in chronically instrumented fetal sheep (127-135 days gestation, term ≈145 days) using laser Doppler flowmetry probes implanted in the parietal cortices. Hypoxia was administered for 30 min by lowering the ewe's inspired oxygen to 10–12 % during an infusion of either saline or theophylline, a non-specific adenosine receptor antagonist. The theophylline infusion was begun 30 min prior to and ended 30 min after the completion of the hypoxic insult. The administration of theophylline had no significant effect on cerebral blood flow during the baseline period. During control hypoxic periods, cerebral blood flow increased by ≈45 %. During theophylline experiments, however, there was no significant increase in cerebral blood flow during hypoxia. In the control experiments, cerebral blood flow returned to baseline levels during the recovery period, while in the theophylline experiments cerebral blood flow fell below baseline levels. We conclude that, in the near-term ovine fetus, adenosine plays a minimal role in the regulation of basal cerebral blood flow. However, these data are strong evidence for the involvement of adenosine in increased fetal cerebral blood flow during an acute hypoxic insult. Finally, adenosine may also play an important role in the maintenance of fetal cerebral blood flow immediately following hypoxic insult.
Insufficient oxygen supply is one of the most common insults to which the fetus is subjected. Because the fetus is unable to alter oxygen availability, cardiovascular mechanisms allowing it to optimize oxygen utilization during hypoxic stress are of utmost importance. The fetal cardiovascular responses to hypoxia include a centralization of blood flow resulting in increased flow to critical organs such as the brain, heart and adrenals, and decreased flow to peripheral organs (for review see Jensen et al. 1999). The acute decrease in blood flow to the periphery, mediated primarily by the systemic release of catecholamines from the adrenal gland and increased sympathetic activity acting on the periphery, directs oxygen delivery to the critical organs (Cohen et al. 1982). However, increases in blood flow to the brain, heart and adrenals during hypoxia appear to be mediated not only by peripheral vasoconstriction but also by decreases in the vascular resistance of these critical organs (Reuss et al. 1982; Iwamoto et al. 1983). In the fetus, the mediators of hypoxic vasodilatation in these organs are not yet known.
Adenosine is a purine nucleoside that has long been established as a potent vasodilator in nearly all vascular beds studied, including the brain (Winn et al. 1981a). In addition, plasma and intracerebral adenosine concentrations correlate inversely with arterial oxygen content in the fetal sheep (Kjellmer et al. 1989; Koos et al. 1994b; Suzuki & Power, 1999), newborn piglet (Laudignon et al. 1991) and adult rat(Winn et al. 1981b; Van Wylen et al. 1986), providing evidence that adenosine is present in vasoactive concentrations during hypoxia. There are also numerous reports on the role of adenosine in regulation of cerebral blood flow during hypoxia in both the adult rat (Morii et al. 1987; Simpson & Phillis, 1991; Coney & Marshall, 1998) and newborn piglet (Laudignon et al. 1990; Park et al. 1995; Bari et al. 1998b). There are conflicting reports, however, since similar studies in newborn piglets showed that the adenosine receptor antagonist theophylline did not attenuate increases in cerebral blood flow during hypoxia (McPhee & Maxwell, 1987).
While there is much evidence implicating adenosine as a regulator of cerebral blood flow in the hypoxic newborn and adult mammals, the evidence in the fetus is circumstantial. Using the pial window technique, Kurth & Wagerle (1992) demonstrated vasodilatory effects of adenosine applied to the cerebral vasculature of the pre-term (0.6-0.7 gestation) and near-term (0.9 gestation) fetal sheep. This, in addition to the fact that adenosine concentrations increase during hypoxia, implicates adenosine as a key mediator of increased cerebral blood flow in the fetus in response to hypoxia.
In the present study, we test the hypothesis that, in the ovine fetus, increases in cerebral blood flow during moderate hypoxia are mediated, at least in part, by adenosine. We employ laser Doppler flowmetry to continuously measure cerebral blood flow of the fetus as previously described (Lan et al. 2000). The effects of moderate hypoxia on cerebral blood flow and cerebral vascular resistance are measured with and without the intravenous administration of theophylline, a non-selective adenosine receptor antagonist.
METHODS
Surgical preparation
Western ewes obtained from Nebeker Ranch (Lancaster, CA, USA) and carrying singleton or twin fetuses were used for the study. Only one of the fetuses was instrumented in ewes carrying twins. Surgical instrumentation was performed at 122–127 days gestation. Following an overnight fast, anaesthesia was induced with 0.5 g thiopental injected into the jugular vein and maintained with 1.5-2.5 % inhaled halothane. A polyvinyl catheter was placed in the femoral vein of the ewe for administration of intravenous fluids (1 l of 0.9 % buffered NaCl) during the surgery, as well as administration of a euthanasia solution at the termination of the experiments. The head and neck of the fetus were exposed through a midline incision in the abdomen of the ewe. Polyvinyl catheters were placed in the brachial artery and advanced to the brachial trunk for blood gas sampling and arterial blood pressure measurement, in the subclavian vein for theophylline administration, and in the amniotic sac for measurement of amniotic fluid pressure and administration of antibiotics to the fetus. The skull of the fetus was exposed with a coronal incision for bilateral placement of laser Doppler flow probes as previously described (Lan et al. 2000). Briefly, two burr holes were drilled bilaterally in the fetal skull approximately 5 mm lateral to the midline and 15 mm posterior to the coronal suture. Laser Doppler flow probes with a diameter of 0.4 mm were embedded in a custom-made mould shaped to fit the contours of the fetal skull. The probes were then inserted through the burr-holes to a depth of 5 mm below the dura, placing the probe tip in the grey matter of parasagittal parietal cortex. The moulds were fixed to the skull with tissue adhesive to minimize movement of the tip of the probe relative to the brain tissue. A thermocouple was inserted into the brachial artery of the fetus together with the polyvinyl catheter, for measurement of core body temperature in order to correct for temperature during arterial blood gas analysis. The fetal scalp and neck incisions were sutured closed and the fetus was returned to the uterus. The uterus and abdomen of the ewe were sutured shut in layers.
All catheters, thermocouple, and laser Doppler flow probes were exteriorized through the maternal flank and held in a protective pouch between experiments. The ewe received 900 000 U of penicillin (i.m.) for the first three postoperative days. The fetus received 500 mg of ampicillin and 40 mg of gentamicin in the amniotic fluid each postoperative day until the animals were killed. Experiments were carried out 3–10 days following the operation, with fetal gestational ages at the time of study ranging from 127 to 135 days. Control and experimental protocols were separated by a period of 48–72 h. Experiments were carried out with the ewe standing in a metabolic cart at room temperature with minimal distractions. The ewe was given access to alfalfa pellets and water throughout each experiment.
Experimental protocol
The control protocol consisted of a 60 min baseline period followed by a 30 min period of hypoxia induced by ventilating the ewe with 10–12 % O2 in a balance of nitrogen. PCO2 was allowed to fall during hypoxia as the respiratory rate of the ewes increased in order to increase oxygen intake. The air mixture was administered into a bag placed over the head of the ewe with a flow of 30 l min−1. The hypoxic period was followed by a 60 min recovery period during which the ewe was allowed to breathe room air. An identical time course was followed for the experimental protocol. However, during the experimental protocol an adenosine receptor blockade was initiated by infusion of a 75 mg bolus (5 ml over ≈30 s) of aminophylline ([theophylline]2-ethylenediamine hydrate; Sigma Chemical Co., St Louis, MO, USA) into the subclavian vein of the fetus at 30 min prior to the start of hypoxia. The blockade was maintained by a continuous infusion of 2.5 mg min−1 until 30 min after the end of the hypoxic period. A placebo (saline) infusion of identical volume (20 ml) was infused during the control protocol from 30 min prior to hypoxia until 30 min after hypoxia.
The sequence of protocol administration and number of sheep studied under each sequence are shown in Table 1. Of the seven sheep studied with theophylline, three were studied under the control protocol first (Sequence 1), and four were studied under the theophylline protocol first (Sequence 2). An additional six sheep were studied under the control protocol and then enrolled in another protocol not discussed in this paper (Sequence 3). There was no significant difference between the control experiments of sheep studied under both the control protocol and theophylline protocol and the experiments of the sheep studied under only the control protocol.
Table 1.
Sequence in which protocols were administered and number of sheep studied in each sequence
| Protocol sequence | No. of sheep | |
|---|---|---|
| Sequence 1 | Control → theophylline | 4 |
| Sequence 2 | Theophylline → control | 3 |
| Sequence 3 | Control only | 6 |
Upon completion of the experiments, the ewe and fetus were killed using a lethal injection of Euthasol (Western Medical Supply, Arcadia, CA, USA), a proprietary euthanasia solution, into the maternal femoral catheter. The location of the probes, thermocouple and catheters was verified. The study protocol was approved by the Loma Linda University Animal Care Research Committee.
Blood sampling
A total of 15 blood samples (1.5 ml) were collected into heparinized syringes from the fetal brachial artery catheter, making the total amount of blood drawn during each experiment amount to less than 10 % of the total fetal blood volume of ≈300 ml. Samples were collected during each experiment at 0, 15, 30, 40, 50 and 60 min during the baseline period, 5, 10, 20 and 30 min during the hypoxic period and 10, 20, 30, 45 and 60 min during the recovery period. The first 0.5 ml was collected for measurement of blood gases using microelectrodes (ABL3, Radiometer, Copenhagen, Denmark) and haemoglobin concentration and saturation using an OSM2 Hemoximeter (Radiometer). The second 0.5 ml was centrifuged to obtain plasma for measurement of glucose and lactate concentrations using a YSI 2700 analyser (Yellow Springs Instruments, Dayton, OH, USA). The third 0.5 ml was added immediately to an equal volume of ice-cold adenosine stop solution (9-erythro-2-(hydroxy-3-nonyl) adenine, 120 μM; dipyridamole, 20 mm; α,β-methylene adenosine-5′ diphosphate, 60 mm; and ethylenediaminetetraacetic acid dipotassium salt, 4.2 mm). The mixture was then centrifuged at 4 °C for 5 min at 1000 g The supernatants of plasma and stop solutions were removed and frozen at -70 °C for subsequent analysis of adenosine as described below.
Adenosine HPLC assay
Plasma samples were deproteinized using an ultrafiltration cone (Amicon; Millipore Corp., Bedford, MA, USA) centrifuged at 2000 g for 1 h at room temperature. Samples of ultrafiltrate were stored at -70 °C until HPLC analysis was carried out as described previously (Yoneyama & Power, 1992). Briefly, 50 μl aliquots of the ultrafiltrate were injected onto a C18 column (Radial-Pak; Waters, Milford, MA, USA), and the absorbance of the eluate was monitored continuously at 254 nm. The moving phase was 0.075 m NH2PO4 in 8 % methanol (pH 6.4) pumped at 1.3 ml min−1. Adenosine was identified by retention time, coelution with standards and peak degradation with adenosine deaminase. In the range studied, peak height was shown to be a linear function of adenosine concentration.
Electronic data acquisition and handling
Cerebral blood flow (CBF) was measured by laser Doppler flowmetry with a Biopac LDF100A laser-Doppler flowmeter (Biopac Systems, Santa Barbara, CA, USA) as previously described (Lan et al. 2000). Arterial blood pressure was measured continuously from the brachial artery. Laser Doppler flowmetry and blood pressure were both sampled at 100 Hz and recorded throughout all experiments by a Macintosh computer using the MP100 analogue-to-digital converter and Acqknowledge software (version 3.0, Biopac Systems). Heart rate was calculated from the pressure wave of the arterial pressure measurements using the Acqknowledge waveform analysis function (Biopac). Cerebral vascular resistance was calculated as (mean brachial arterial blood pressure)/(CBF), with the assumption that brachial arterial pressure was an accurate representation of cerebral perfusion pressure and both blood pressure and CBF calculated as a percentage of baseline values. Following the experiments, spikes in the laser Doppler data with an increase of > 100 % in less than 1 s were judged as movement artifacts and were removed. The laser Doppler and blood pressure data were then re-sampled in 3 min averages and the values exported to a spreadsheet for analysis. Laser Doppler flowmetry provides a relative, not absolute measure of flow. Therefore, all the cerebral blood flow values for the baseline period of each experiment were averaged and considered as 100 %, with measurements during the hypoxia and recovery periods expressed as a percentage of the baseline values. After conversion of the laser Doppler blood perfusion units to a percentage of baseline values, CBF for each fetus was taken as an average of the laser Doppler data from the two laser Doppler probes implanted in each fetus with the exception of three fetuses in which one of the two probes was dysfunctional. There was no significant difference between laser Doppler measurements taken from the right and left sides of the brain.
Statistical analyses
Data are presented as means ± s.e.m. The significance of changes in cerebral blood flow and cerebral vascular resistance over time were evaluated using a one-way ANOVA with repeated measures by using Fischer's test of least significant difference for multiple-comparisons test. The significance of differences between the control and theophylline protocols in cerebral blood flow, blood pressure and heart rate were evaluated using a two-way ANOVA with repeated measures and Fischer's test.
The means of arterial blood pressure, heart rate, haemoglobin, haemoglobin saturation, arterial PO2 and PCO2, plasma glucose, lactate and adenosine were calculated for the baseline, drug or saline infusion, hypoxia and recovery periods for both control and experimental protocols. Values deemed significantly different than those of the baseline period were detected using one-way ANOVA with Dunnett's post hoc test. Significant differences between the control and theophylline experiments were detected using one-way ANOVA with Tukey's post hoc test. Statistical significance was assumed at P < 0.05.
The statistical software DATAMSTR (Courtesy of R. A. Brace, UC San Diego, CA, USA) was used to perform one- and two-way ANOVAs on data for cerebral blood flow, blood pressure and heart rate. Graphpad Prism (Graphpad Software Inc., San Diego, CA, USA) was used to perform ANOVAs on data presented in Table 1.
RESULTS
Cerebral blood flow and cerebral vascular resistance responses
Cerebral blood flow for both control and theophylline experiments is shown in Fig. 1. There was no significant difference between the control experiments and the theophylline experiments during the 1 h baseline period. There was also no significant change in cerebral blood flow with the initiation of theophylline infusion during the baseline period. In the control experiments, cerebral blood flow increased progressively throughout the hypoxic period, reaching 144 ± 8 % of baseline values at the end of the 30 min hypoxic period. In contrast, there was no significant increase in cerebral blood flow during hypoxia in the animals treated with theophylline. In the control experiments, cerebral blood flow returned to baseline levels within the first 15 min of the recovery period. Although there was a tendency for cerebral blood flow to increase during the second 30 min of the recovery period, it did not vary significantly from baseline values. In the theophylline experiments, cerebral blood flow fell progressively during the first 20 min of the recovery period, remaining somewhat lower than baseline values for the remainder of the experiment.
Figure 1. Effects of hypoxia on cerebral blood flow and cerebral vascular resistance.
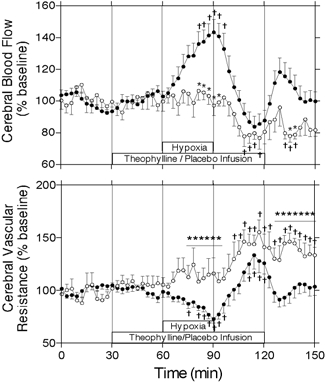
Cerebral blood flow and cerebral vascular resistance ± s.e.m. during baseline, hypoxia and recovery periods as measured by laser Doppler flowmetry in control (•, n = 12) and theophylline (○, n = 7) experiments. Cerebral vascular resistance was calculated as arterial blood pressure divided by cerebral blood flow (percentage of the baseline). * Significant difference between control and theophylline groups (P < 0.05, two-way ANOVA); † significant difference from mean of pre-infusion period (P < 0.05, one-way ANOVA).
Cerebral vascular resistance for both control and theophylline experiments is shown in Fig. 1. Infusion of theophylline had no significant effect on cerebral vascular resistance during the baseline period. Cerebral vascular resistance decreased from baseline progressively during the hypoxic period in the control experiments, but remained constant throughout the hypoxic period in the theophylline experiments. During the recovery period, cerebral vascular resistance returned to baseline levels in the control experiments, but increased above baseline levels in the theophylline experiments. Cerebral vascular resistance was significantly greater in the theophylline experiments during the final 15 min of the hypoxic period and the final 20 min of the recovery period.
Mean arterial blood pressure and heart rate
Mean arterial blood pressure (MABP) and heart rate are presented in Fig. 2. In the control experiments, MABP increased from an average of 45 ± 0 mmHg in the baseline period to a peak of 51 ± 3 mmHg at the end of the hypoxic period, and then returned to baseline values with an average of 45 ± 0 mmHg during the recovery period. In the theophylline experiments, MABP increased slightly from an average of 47 ± 1 mmHg to an average of 50 ± 1 mmHg upon administration of theophylline during the baseline period, the peak 3 min average of 51 ± 2 mmHg being significantly greater than baseline values. During hypoxia, MABP increased further in the theophylline experiments, reaching a peak of 57 ± 3 mmHg. In the recovery period, MABP remained elevated above baseline values with an average of 53 ± 1 mmHg.
Figure 2. Effects of hypoxia on mean arterial blood pressure and heart rate.
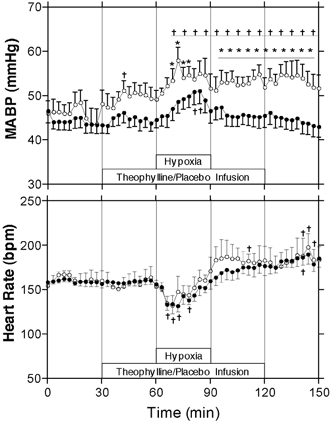
Arterial blood pressure and heart rate ± s.e.m. during baseline, hypoxia and recovery periods. * Significant difference between control (•, n = 12) and theophylline (○, n = 7) groups (P < 0.05, two-way ANOVA); † significant difference from mean of pre-infusion period (P < 0.05, one-way ANOVA).
Heart rate did not change significantly with the administration of theophylline during the baseline period. In addition, there was no significant difference in heart rate between the control and theophylline groups for any stage of the experiment. In both the control and theophylline experiments, heart rate decreased from a baseline of ≈165 to a nadir of ≈140 beats min−1 during the first 15 min of the hypoxic period. Heart rate then increased progressively to an average of 184 ± 1 beats min−1 during the final 30 min of the recovery period.
Fetal blood gas, pH, haemoglobin, oxygen content, glucose, lactate and adenosine values
Arterial PO2, oxygen content and oxygen delivery are shown in Fig. 3. Oxygen delivery was calculated as the product of arterial oxygen content at each sampling time point (expressed as a percentage of the average baseline arterial content) multiplied by the per cent baseline value of CBF at the time the sample was collected. PCO2, haemoglobin content, pH, and glucose, lactate and adenosine concentrations are shown in Table 2 as mean values for the baseline, baseline with saline or theophylline infusion, hypoxia and recovery periods. PO2 decreased significantly from baseline to about half of normal levels during the hypoxic period in both the control and theophylline groups, with no significant differences between the control and theophylline experiments. There was also a significant decrease from baseline in PCO2 during the hypoxic period in both the control and theophylline experiments in addition to PCO2 values in the theophylline experiments being significantly higher compared to the control experiments.
Figure 3. Effects of hypoxia on arterial PO2, oxygen content and cerebral oxygen delivery.
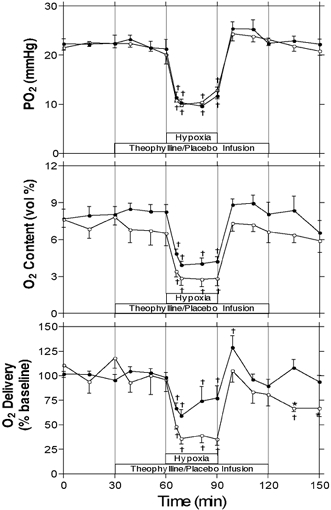
Arterial PO2, oxygen content and cerebral oxygen delivery ± s.e.m. during baseline, hypoxia and recovery periods. Cerebral oxygen delivery was calculated as the product of arterial oxygen content (percentage of baseline) and laser Doppler flowmetry (percentage of baseline) at the time the blood sample was collected. * Significant difference between control (•, n = 12) and theophylline (○, n = 7) groups (P < 0.05, two-way ANOVA); † significant difference from mean of pre-infusion period (P < 0.05, one-way ANOVA).
Table 2.
Data (mean ±S.E.M.) for blood and plasma variables during the four key protocol time periods
| Variable | Group | Baseline | s.e.m. | Theophylline or saline infusion | s.e.m. | Hypoxia | s.e.m. | Recovery | s.e.m. |
|---|---|---|---|---|---|---|---|---|---|
| PCO2(mmHg) | Control | 46.7 | 0.5 | 47.3 | 0.5 | 42.3b | 0.6 | 46.6 | 0.5 |
| Theophylline | 49.2 | 0.7 | 48.5 | 4.2 | 45.3bd | 0.9 | 47.2 | 0.5 | |
| Hb (gdl−1) | Control | 9.84 | 0.24 | 10.10 | 0.24 | 10.78a | 0.25 | 9.74 | 0.22 |
| Theophylline | 9.38 | 0.21 | 9.34 | 1.02 | 10.30b | 0.20 | 9.64 | 0.17 | |
| pH | Control | 7.33 | 0.00 | 7.34 | 0.00 | 7.34 | 0.01 | 7.27b | 0.01 |
| Theophylline | 7.32 | 0.01 | 7.33 | 0.00 | 7.30c | 0.01 | 7.24bc | 0.01 | |
| Glucose (mg dl−1) | Control | 18.7 | 0.7 | 20.4 | 0.9 | 23.2 | 1.1 | 26.4b | 1.3 |
| Theophylline | 18.2 | 1.0 | 19.1 | 1.9 | 25.2b | 1.5 | 28.6b | 1.4 | |
| Lactate (mmol l−1) | Control | 1.2 | 0.1 | 1.4 | 0.2 | 4.5b | 0.5 | 5.3b | 0.4 |
| Theophylline | 1.3 | 0.1 | 1.4 | 0.1 | 4.6b | 0.7 | 7.2b,c | 0.6 | |
| Adenosine (μM) | Control | 1.11 | 0.21 | 1.19 | 0.25 | 1.33 | 0.34 | 1.27 | 0.35 |
| Theophylline | 1.13 | 0.16 | 1.09 | 0.16 | 1.29 | 0.20 | 1.21 | 0.24 |
Significant difference from baseline (P < 0.05).
Significant difference from baseline (P < 0.01).
Significant difference from corresponding control value (P < 0.05).
Significant difference from corresponding control value (P < 0.01).
Haemoglobin concentrations increased significantly from baseline during the hypoxic period in both the control and theophylline experiments. There was no significant difference between the control and theophylline values for haemoglobin concentration. The pH values for both the control and theophylline experiments were significantly less than baseline values during the recovery period. In addition, the pH values of the theophylline experiments during the hypoxic and recovery periods were significantly less than those of the control experiments for the same period. Arterial oxygen content was significantly decreased from baseline values during the hypoxic period in both the control and theophylline experiments.
Glucose values were elevated significantly above baseline during the recovery period for control experiments, and during the hypoxia and recovery periods for the theophylline experiments. There was no significant difference in glucose values between the control and theophylline experiments. Lactate values during the hypoxia and recovery periods were significantly greater than were those of the baseline periods for both the control and the theophylline experiments. In addition, lactate values during the recovery period were significantly greater in the theophylline experiments than were those of the control experiments. There was no significant change in plasma adenosine concentrations during hypoxia in either the control or theophylline experiments.
Discussion
The principal finding of the present study is that systemic infusion of theophylline, a non-selective adenosine receptor antagonist, abolishes increases in cerebral blood flow during hypoxia in the near-term ovine fetus. In addition, infusion of theophylline during basal conditions has no effect on fetal cerebral blood flow or cerebral vascular resistance. Finally, cerebral vascular resistance is increased by the infusion of theophylline following a hypoxic insult indicating that the post-hypoxic hyperaemia that is seen in these experiments is mediated by adenosine.
Adenosine is a purine nucleotide that can be produced by the 5′-adenosine monophosphate (5′-AMP) either inside or outside the cell. In the fetal sheep, increases greater than twofold in intracerebral adenosine concentrations during hypoxia have been reported and are proposed to be due primarily to increased extracellular metabolism of 5′-AMP (Koos et al. 1997). Increases in plasma adenosine as high as twofold have been reported during hypoxia (Koos & Doany, 1991), but some reports are significantly less than this (Yoneyama & Power, 1992). The present study is in agreement with the latter study, as there was no significant increase in plasma adenosine during hypoxia. Since adenosine transport across the blood-brain barrier is mediated by a saturable, facilitative transport system, it is possible for increases in plasma adenosine concentration to be reflected in the central nervous system (Koos & Doany, 1991). However, the greater magnitude of increases in adenosine concentrations in the brain relative to the plasma suggests that, during hypoxia, increases in cerebral adenosine concentrations are due to a local paracrine-type effect. In addition, intravenous infusion of adenosine to the normoxic near-term fetal sheep does not result in increased oxygen delivery to the head, also suggesting that hypoxic increases in cerebral blood flow are not mediated by increased plasma adenosine concentrations alone (Newman et al. 2001).
Information regarding specific subtype and location of adenosine receptors responsible for mediating hypoxic cerebral vasodilatation in adult and newborn animals is sparse. Several investigators have attempted to determine which side of the blood-brain barrier the vasoactive adenosine receptors are located on. Studies using the pial window in the adult rat indicate that neuronal stimulation results in vasodilatation mediated by A2A receptors on the inside of the blood-brain barrier (Meno et al. 2001). However, others have reported that cerebral vasodilatation may be mediated by A2B receptors on the luminal surface of the blood-brain barrier (Ngai & Winn, 1993; Coney & Marshall, 1998). In light of this conflicting information, we chose the non-selective adenosine receptor antagonist theophylline in order to achieve blockade of all adenosine receptors irrespective of subtype or location. Theophylline antagonizes all adenosine receptor subtypes (Olah & Stiles, 1995), is membrane permeable and readily crosses the blood-brain barrier when administered intravenously (McPhee & Maxwell, 1987; Morii et al. 1987).
Our finding that theophylline completely blocks increases in cerebral blood flow during hypoxia implies a strong role for adenosine in hypoxic cerebral vasodilatation in the fetus. Although, to our knowledge, this is the first report on the role of adenosine in hypoxic cerebral hyperaemia in the fetus, these results are consistent with many previous reports in adult and newborn animals that implicate adenosine as being partially or totally responsible. In the adult rat, attenuation of hypoxic increases in cerebral blood flow has been observed following intraperitoneal administration of theophylline (Morii et al. 1987). Bari and coworkers observed blockade of hypoxic cerebral vasodilatation in the piglet using the pial window technique and either topical (Bari et al. 1998b) or intravenous (Bari et al. 1998a) administration of theophylline. Also using the newborn piglet, Laudignon et al. (1990) observed attenuation of hypoxic cerebral hyperaemia by intravenous infusion of 8-phenyltheophylline (8-PT) and complete blockade by intracerebroventricular infusion of 8-PT. In contrast, McPhee & Maxwell (1987) observed no effect of intravenous theophylline on hypoxic increases in cerebral blood flow in the piglet. The conflicting results of McPhee & Maxwell (1987) may have been due to insufficient concentrations of theophylline at the receptors mediating vasodilatation, since the dose of theophylline administered was only slightly greater than the dose of the more potent 8-PT required in the above study by Laudignon et al. (1990) and half of the dose of theophylline administered by Bari et al. (1998a).
The possibility exists that the effects of theophylline observed in the present study are mediated through the vasopressin system. Vasopressin causes cerebral vasodilatation in the fetal sheep and its plasma concentrations increase during acute hypoxia resulting in at least some of the hypoxic increase in cerebral blood flow (Eisenbach et al. 1992). Increases in plasma vasopressin concentrations during acute hypoxia are attenuated by intravenous infusion of the adenosine receptor antagonist 8-sulfophenyltheophylline (8-SPT) (Koos et al. 1994a). Therefore, it is possible that infusion of theophylline in the present study attenuated increases in plasma vasopressin concentrations during hypoxia, thereby blunting the dilatory actions of vasopressin on the cerebral vasculature.
The possibility also exists that adenosine mediates hypoxic increases in cerebral blood flow by decreasing central venous pressure, thereby increasing cerebral perfusion pressure. As stated earlier, calculation of cerebrovascular resistance in the present study was based solely on mean arterial blood pressure with the assumption that changes in central venous pressure were insignificant during hypoxia. Therefore, the possibility that theophylline administration resulted in significantly greater central venous pressures during hypoxia must also be considered.
The administration of non-selective adenosine receptor antagonists appears to have minimal effect on basal cerebrovascular tone in the adult rat (Coney & Marshall, 1998; Meno et al. 2001), newborn piglet (Laudignon et al. 1990; Bari et al. 1998a), and preterm and term fetal sheep (Kurth & Wagerle, 1992), suggesting that adenosine does not play an important role in the maintenance of basal cerebral blood flow. The findings of the present study are in agreement with these previous reports since cerebrovascular resistance or cerebral blood flow were not affected by theophylline infusion during the baseline period despite an increase in MABP.
In the fetal sheep, cerebral blood flow typically returns to basal levels during the hour immediately following a hypoxic insult such as the one administered in the present study (Jensen et al. 1999; Lan et al. 2000). Intracerebral adenosine concentrations in the fetus also decrease during recovery, although they may remain slightly higher than baseline values during the hour following a hypoxic insult (Koos et al. 1994b). Theophylline administration in the present study resulted in cerebral blood flow slightly less than baseline values and cerebrovascular resistance significantly greater than baseline values during recovery, indicating that adenosine may still play an active vasodilatory role immediately following hypoxic stress.
During hypoxic stress, the fetal carotid body mediates increases in arterial blood pressure and peripheral vascular resistance, a decrease in heart rate and increases in plasma glucose and lactate concentrations (Giussani et al. 1993). All of these responses are attenuated by intravenous infusion 8-SPT (Koos et al. 1995; Giussani et al. 2001) or the selective A2A antagonist ZM-241385 (Koos & Maeda, 2001). In contrast to these reports, administration of theophylline in the present study did not attenuate any of the above responses. It is possible that the dose and distribution of theophylline was such that it achieved concentrations sufficient to block cerebral adenosine receptors that mediate hypoxic increases in cerebral blood flow without antagonizing the actions of the adenosine receptors responsible for the carotid sinus-mediated responses. Conversely, because theophylline is known to have pharmacological effects other than blockade of adenosine receptors, such as inhibition of phosphodiesterase activity (Fredholm & Sydbom, 1980), it is also conceivable that failure to attenuate these responses was due to non-specific effects of theophylline on the chemoreceptor responses. However, the latter does not seem likely since a 50 % inhibition of phosphodiesterase activity requires a theophylline concentration of ≈1 mm (Fredholm & Sydbom, 1980) and we have reported previously that administration of theophylline at a rate 25 % less than the dose administered in the present study resulted in plasma concentrations of only 150 μM (Ball et al. 1996). In addition, Koos & Matsuda (1990) have reported previously that administration of theophylline to the fetal sheep at a rate ≈30 % greater than the dose administered in the present study resulted in plasma concentrations well below 1 mm, with significant attenuation of the effects of intravenous administration of adenosine. Finally, we have found that upon stepwise increases in the infusion rate of theophylline to the fetal sheep, increases in arterial blood pressure are noted at the infusion rate used in the present study, while decreases in arterial blood pressure, presumably due to phosphodiesterase inhibition, do not occur until infusion rates approximately 20-fold greater than that used in the present study (G. G. Power & K. T. Ball, unpublished data).
Although Laser Doppler flowmetry has been used for the measurement of blood flow in living tissue for over 30 years, it has only recently been adapted to the study of cerebral blood flow in the fetus (Lan et al. 2000). Even more recently, Muller et al. (2002) have reported a close correlation between laser Doppler flowmetry and fluorescent microspheres in the cerebral cortex of the fetal sheep and demonstrated the usefulness of laser Doppler flowmetry in the study of the autoregulatory mechanisms of the fetal brain. In addition, they also reported gliosis of only 0.35 ± 0.06 mm in thickness around the tip of the probe, indicating that artifacts in flow measurement due to the traumatic insertion of the probe into the cortex are most likely to be insignificant. Even though laser Doppler flowmetry provides only a relative measurement of blood flow in a tissue volume of only ≈2 mm3 (Stern et al. 1977), the data in the present study further demonstrate the usefulness of this technique for continuous measurements of fetal cerebral blood flow with the ability to perform multiple experiments in each animal.
In summary, intravenous administration of theophylline, a non-selective adenosine receptor antagonist, abolishes normal increases in cerebral blood flow in response to acute hypoxia in the near-term ovine fetus. We therefore conclude that adenosine plays a major role in mediating hypoxic increases in cerebral blood flow by effecting decreases in cerebrovascular resistance. In addition, theophylline had no effect on basal cerebral blood flow or cerebrovascular resistance, indicating that adenosine is not a significant mediator of basal cerebral blood flow. Finally, theophylline administration resulted in a significant decrease in cerebral blood flow and cerebral vascular resistance during a 1 h recovery from hypoxia, indicating adenosine may also be an important regulator of cerebral blood flow during the recovery from a hypoxic insult.
Acknowledgments
The authors thank Shannon Bragg for expert technical assistance. This study was supported in part by USPHS award HL65494 and in part by NMTB and the Department of the Army, Cooperative Agreement Number DAMD17-97-2-7016. The information does not necessarily reflect the position or policy of the US government or NMTB and no official endorsement should be inferred.
References
- Ball KT, Gunn TR, Gluckman PD, Power GG. Suppressive action of endogenous adenosine on ovine fetal nonshivering thermogenesis. Journal of Applied Physiology. 1996;81:R2393–2398. doi: 10.1152/jappl.1996.81.6.2393. [DOI] [PubMed] [Google Scholar]
- Bari F, Louis TM, Busija DW. Effects of ischemia on cerebral arteriolar dilation to arterial hypoxia in piglets. Stroke. 1998a;29:222–227. doi: 10.1161/01.str.29.1.222. [DOI] [PubMed] [Google Scholar]
- Bari F, Thore CR, Louis TM, Busija DW. Inhibitory effects of hypoxia and adenosine on N-methyl-d-aspartate-induced pial arteriolar dilation in piglets. Brain Research. 1998b;780:237–244. doi: 10.1016/s0006-8993(97)01196-7. [DOI] [PubMed] [Google Scholar]
- Cohen WR, Piasecki GJ, Jackson BT. Plasma catecholamines during hypoxemia in fetal lamb. American Journal of Physiology. 1982;243:R520–525. doi: 10.1152/ajpregu.1982.243.5.R520. [DOI] [PubMed] [Google Scholar]
- Coney AM, Marshall JM. Role of adenosine and its receptors in the vasodilatation induced in the cerebral cortex of the rat by systemic hypoxia. Journal of Physiology. 1998;509:507–518. doi: 10.1111/j.1469-7793.1998.507bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbach JC, Tong C, Stump DA, Block SM. Vasopressin and fetal cerebrovascular regulation. American Journal of Physiology. 1992;263:R376–381. doi: 10.1152/ajpregu.1992.263.2.R376. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Sydbom A. Are the anti-allergic actions of theophylline due to antagonism at the adenosine receptor. Agents and Actions. 1980;10:145–147. doi: 10.1007/BF02024198. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Gardner DS, Cox DT, Fletcher AJ. Purinergic contribution to circulatory, metabolic, and adrenergic responses to acute hypoxemia in fetal sheep. American Journal of Physiology - Renal Physiology. 2001;280:R678–685. doi: 10.1152/ajpregu.2001.280.3.R678. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Spencer JA, Moore PJ, Bennet L, Hanson MA. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. Journal of Physiology. 1993;461:431–449. doi: 10.1113/jphysiol.1993.sp019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto HS, Rudolph AM, Mirkin BL, Keil LC. Circulatory and humoral responses of sympathectomized fetal sheep to hypoxemia. American Journal of Physiology. 1983;245:H767–772. doi: 10.1152/ajpheart.1983.245.5.H767. [DOI] [PubMed] [Google Scholar]
- Jensen A, Garnier Y, Berger R. Dynamics of fetal circulatory responses to hypoxia and asphyxia. European Journal of Obstetrics and Gynecological Reproductive Biology. 1999;84:155–172. doi: 10.1016/s0301-2115(98)00325-x. [DOI] [PubMed] [Google Scholar]
- Kjellmer I, Andiné P, Hagberg H, Thiringer K. Extracellular increase of hypoxanthine and xanthine in the cortex and basal ganglia of fetal lambs during hypoxia-ischemia. Brain Research. 1989;478:241–247. doi: 10.1016/0006-8993(89)91504-7. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Chau A, Ogunyemi D. Adenosine mediates metabolic and cardiovascular responses to hypoxia in fetal sheep. Journal of Physiology. 1995;488:761–766. doi: 10.1113/jphysiol.1995.sp021007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos BJ, Doany W. Role of plasma adenosine in breathing responses to hypoxia in fetal sheep. Journal of Developmental Physiology. 1991;16:81–85. [PubMed] [Google Scholar]
- Koos BJ, Kruger L, Murray TF. Source of extracellular brain adenosine during hypoxia in fetal sheep. Brain Research. 1997;778:439–442. doi: 10.1016/s0006-8993(97)01207-9. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Maeda T. Adenosine A(2A). receptors mediate cardiovascular responses to hypoxia in fetal sheep. American Journal of Physiology - Heart and Circulatory Physiology. 2001;280:H83–89. doi: 10.1152/ajpheart.2001.280.1.H83. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Mason BA, Ervin MG. Adenosine mediates hypoxic release of arginine vasopressin in fetal sheep. American Journal of Physiology. 1994a;266:R215–220. doi: 10.1152/ajpregu.1994.266.1.R215. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Mason BA, Punla O, Adinolfi AM. Hypoxic inhibition of breathing in fetal sheep: relationship to brain adenosine concentrations. Journal of Applied Physiology. 1994b;77:2734–2739. doi: 10.1152/jappl.1994.77.6.2734. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Matsuda K. Fetal breathing, sleep state, and cardiovascular responses to adenosine in sheep. Journal of Applied Physiology. 1990;68:489–495. doi: 10.1152/jappl.1990.68.2.489. [DOI] [PubMed] [Google Scholar]
- Kurth CD, Wagerle LC. Cerebrovascular reactivity to adenosine analogues in 0.6–0.7 gestation and near-term fetal sheep. American Journal of Physiology. 1992;262:H1338–1342. doi: 10.1152/ajpheart.1992.262.5.H1338. [DOI] [PubMed] [Google Scholar]
- Lan J, Hunter CJ, Murata T, Power GG. Adaptation of laser-Doppler flowmetry to measure cerebral blood flow in the fetal sheep. Journal of Applied Physiology. 2000;89:1065–1071. doi: 10.1152/jappl.2000.89.3.1065. [DOI] [PubMed] [Google Scholar]
- Laudignon N, Farri E, Beharry K, Aranda JV. Rapid effects of hypoxia on the cerebrospinal fluid levels of adenosine and related metabolites in newborn and one-month-old piglets. Biology of the Neonate. 1991;59:54–59. doi: 10.1159/000243322. [DOI] [PubMed] [Google Scholar]
- Laudignon N, Farri E, Beharry K, Rex J, Aranda JV. Influence of adenosine on cerebral blood flow during hypoxic hypoxia in the newborn piglet. Journal of Applied Physiology. 1990;68:1534–1541. doi: 10.1152/jappl.1990.68.4.1534. [DOI] [PubMed] [Google Scholar]
- McPhee AJ, Maxwell GM. The effect of theophylline on regional cerebral blood flow responses to hypoxia in newborn piglets. Pediatric Research. 1987;21:573–578. doi: 10.1203/00006450-198706000-00014. [DOI] [PubMed] [Google Scholar]
- Meno JR, Crum AV, Winn HR. Effect of adenosine receptor blockade on pial arteriolar dilation during sciatic nerve stimulation. American Journal of Physiology - Heart and Circulatory Physiology. 2001;281:H2018–2027. doi: 10.1152/ajpheart.2001.281.5.H2018. [DOI] [PubMed] [Google Scholar]
- Morii S, Ngai AC, Ko KR, Winn HR. Role of adenosine in regulation of cerebral blood flow: effects of theophylline during normoxia and hypoxia. American Journal of Physiology. 1987;253:H165–175. doi: 10.1152/ajpheart.1987.253.1.H165. [DOI] [PubMed] [Google Scholar]
- Muller T, Lohle M, Schubert H, Bauer R, Wicher C, Antonow-Schlorke I, Sliwka U, Nathanielsz PW, Schwab M. Developmental changes in cerebral autoregulatory capacity in the fetal sheep parietal cortex. Journal of Physiology. 2002;539:957–967. doi: 10.1113/jphysiol.2001.012590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JP, Peebles DM, Hanson MA. Adenosine produces changes in cerebral hemodynamics and metabolism as assessed by near-infrared spectroscopy in late-gestation fetal sheep in utero. Pediatric Research. 2001;50:217–221. doi: 10.1203/00006450-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Winn HR. Effects of adenosine and its analogues on isolated intracerebral arterioles. Extraluminal and intraluminal application. Circulatory Research. 1993;73:448–457. doi: 10.1161/01.res.73.3.448. [DOI] [PubMed] [Google Scholar]
- Olah ME, Stiles GL. Adenosine receptor subtypes: characterization and therapeutic regulation. Annual Reviews in Pharmacology and Toxicology. 1995;35:581–606. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- Park TS, Gonzales ER, Shah AR, Gidday JM. Hypoglycemia selectively abolishes hypoxic reactivity of pial arterioles in piglets: role of adenosine. American Journal of Physiology. 1995;268:H871–878. doi: 10.1152/ajpheart.1995.268.2.H871. [DOI] [PubMed] [Google Scholar]
- Reuss ML, Parer JT, Harris JL, Krueger TR. Hemodynamic effects of alpha-adrenergic blockade during hypoxia in fetal sheep. American Journal Obstetrics and Gynecology. 1982;142:410–415. doi: 10.1016/s0002-9378(16)32381-x. [DOI] [PubMed] [Google Scholar]
- Simpson RE, Phillis JW. Adenosine deaminase reduces hypoxic and hypercapnic dilatation of rat pial arterioles: evidence for mediation by adenosine. Brain Research. 1991;553:305–308. doi: 10.1016/0006-8993(91)90839-n. [DOI] [PubMed] [Google Scholar]
- Stern MD, Lappe DL, Bowen PD, Chimosky JE, Holloway GA, Keiser HR, Bowman RL. Continuous measurement of tissue blood flow by laser Doppler spectroscopy. American Journal of Physiology. 1977;232:H441–448. doi: 10.1152/ajpheart.1977.232.4.H441. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Power GG. Role of adenosine in regulation of brain temperature in fetal sheep. American Journal of Obstetrics and Gynecology. 1999;181:681–687. doi: 10.1016/s0002-9378(99)70513-2. [DOI] [PubMed] [Google Scholar]
- Van Wylen DG, Park TS, Rubio R, Berne RM. Increases in cerebral interstitial fluid adenosine concentration during hypoxia, local potassium infusion, and ischemia. Journal of Cerebral Blood Flow and Metabolism. 1986;6:522–528. doi: 10.1038/jcbfm.1986.97. [DOI] [PubMed] [Google Scholar]
- Winn HR, Rubio GR, Berne RM. The role of adenosine in the regulation of cerebral blood flow. Journal of Cerebral Blood Flow and Metabolism. 1981a;1:239–244. doi: 10.1038/jcbfm.1981.29. [DOI] [PubMed] [Google Scholar]
- Winn HR, Rubio GR, Berne RM. Brain adenosine concentration during hypoxia in rats. American Journal of Physiology. 1981b;241:H235–242. doi: 10.1152/ajpheart.1981.241.2.H235. [DOI] [PubMed] [Google Scholar]
- Yoneyama Y, Power GG. Plasma adenosine and cardiovascular responses to dipyridamole in fetal sheep. Journal of Developmental Physiology. 1992;18:203–209. [PubMed] [Google Scholar]


