Figure 2. Phosphorylation of EGFP-tagged kNBC1 mutants by PKA and characterization of kNBC1:CAII binding.
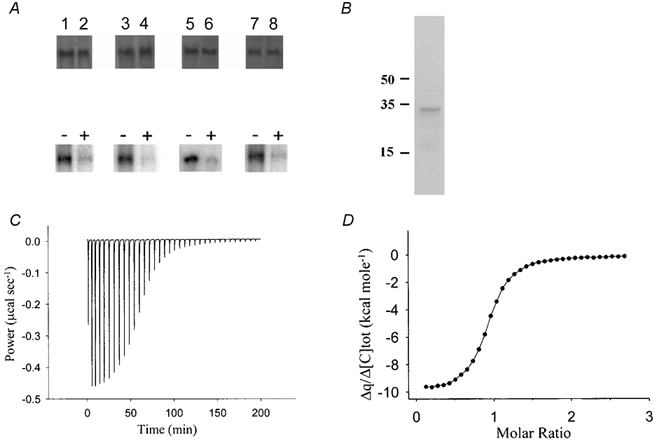
Upper panel in A, Western blots of kNBC1 used in in vitro phosphorylation assays. Treatment of the cells with 8-Br-cAMP did not affect the amount of protein expressed. Lower panel in A, in vitro phosphorylation assays of kNBC1-NNNN (lanes 1 and 2), kNBC1-DNNN (lanes 3 and 4), kNBC1-DNND (lanes 5, and 6) and kNBC1-NNDD (lanes 7 and 8) isolated from untreated mPCT cells (lanes 1, 3, 5 and 7) or cells treated with 100 μm 8-Br-cAMP (lanes 2, 4, 6 and 8) for 15 min. Immunoprecipitated EGFP-kNBC1 mutants were eluted from protein A-Sepharose beads and then phosphorylated in vitro using the PKA catalytic subunit and [γ-32P]ATP. Representative experiments are shown. B, Western blot of mPCT cells with anti-CAII showing the presence of CAII in these cells. Molecular weight labels are in kDa units. C, calorimetric titration of CAII with kNBC1-Ct. Heat changes associated with titration of CAII with kNBC1-Ct. D, binding isotherm and best-fit curve for peak area integrals shown in C. N (number of binding sites) = 1 ± 0.06; KD (dissociation constant) = 160 ± 10 nm.
