Figure 6. Colocalization of XOR, Btn and ADPH on milk lipid globule membranes.
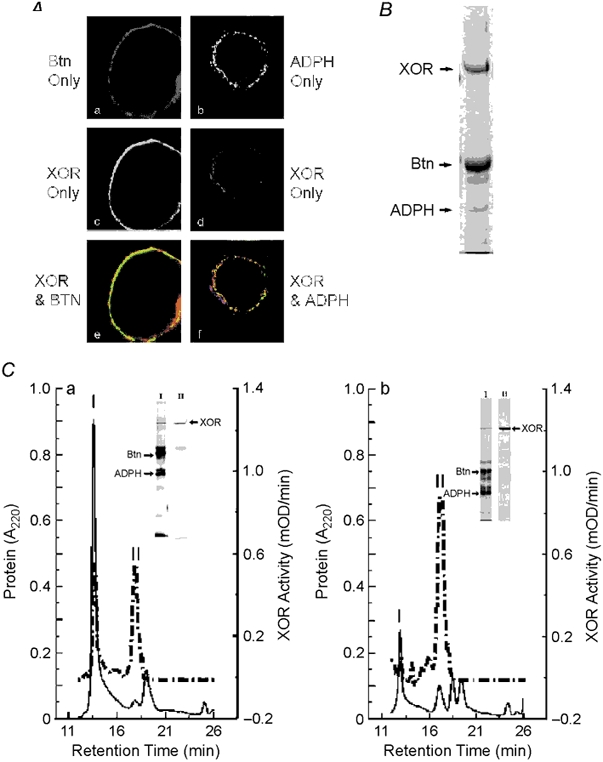
A, XOR was separately immunolocalized with Btn or ADPH in paraformaldehyde-fixed mouse milk. XOR was detected with FITC-labelled antibodies (green) and Btn and ADPH were detected with cy3-labelled antibodies (red) as described. a, Btn only staining; b, XOR only staining; c, merged image of XOR and Btn staining; d, ADPH only staining; e, XOR only staining; f, merged image of XOR and ADPH staining. Note that XOR is largely localized internal to Btn and that all ADPH staining appears to overlap with XOR, as shown by the yellow areas. B, Coomassie-blue-stained gel of mouse milk lipid droplet membrane proteins separated by SDS-PAGE. XOR, Btn and ADPH bands are indicated; their identities were verified by N-terminal microsequence analysis. C, size-exclusion chromatography (SEC) analysis of interactions between XOR, Btn and ADPH isolated from milk lipid globule membranes. Panel a shows the elution properties of XOR enzymatic activity (dashed line) and 220 nm absorbing material (continuous line) following SEC of Triton-X-100-solubilized milk lipid globule membrane proteins. The insets show silver-stained gels of proteins in peaks I and II separated by SDS-PAGE. The identities of XOR, Btn and ADPH bands were confirmed by N-terminal microsequence analysis and are indicated. XOR bands in peaks I and II are indicated by the arrow at the right of the peak II lane. Panel b shows the elution properties of XOR, Btn and ADPH after incubating peak I with 5 mm DTT. XOR activity and A220 nm absorbance are indicated by the dashed and continuous lines, respectively. Silver-stained gels of the proteins in peaks I and II are shown in the insets and the positions of XOR, Btn and ADPH are indicated as above.
