Abstract
Voluntary contractions induce thixotropic changes in intrafusal muscle fibres and hence, by induction or removal of ‘slack’, the background discharge and sensitivity of spindle endings to stretch is altered. This study assessed whether such changes also altered the ‘excitability’ of the motor cortex. Eleven subjects performed a series of voluntary conditioning contractions of the wrist flexors designed to remove slack in the intrafusal fibres (contract and test at intermediate length, termed ‘contract-test’) or to introduce slack (contract at long length and test at intermediate length, termed ‘contract-long’). Surface electromyographic recordings were made from one wrist flexor, flexor carpi radialis. Subjects relaxed after each contraction, and 10 s later a test stimulus was applied to elicit a tendon tap response, H-reflex, or motor-evoked potential (MEP) to transcranial magnetic stimulation in the flexor carpi radialis. Each of the three test stimuli was applied during 15 consecutive pairs of contractions (‘contract-long’ and ‘contract-test’). Three subjects repeated the protocol using transmastoid electrical stimulation as the test stimulus to evoke a cervicomedullary motor-evoked potential (CMEP). For the group of subjects, after conditioning contractions designed to induce slack there was a significant reduction in the amplitude of the tendon reflex, no significant change in the H-reflex, and a small but significant reduction in the amplitude of the MEP. In one subject the CMEP was significantly reduced, while it was unchanged in two others. In the absence of corresponding changes in the H-reflex (or CMEP), changes in the size of the response to motor cortical stimulation suggest that the level of motor cortical ‘excitability’ changes according to naturally induced variations in the discharge of muscle spindle afferents.
Some changes in the stretch sensitivity of muscle spindle endings depend on the history of intrafusal muscle contraction. These ‘after effects’ result from thixotropic changes of the intrafusal sarcomeres (for review Proske et al. 1993). Conditioning voluntary contractions can be used to elicit thixotropic changes in intrafusal fibres. If a muscle contracts at a given length, stable bonds form between some actin and myosin filaments immediately after the contraction. If the muscle is then passively shortened it falls slack. Slack within the intrafusal fibres reduces the discharge and sensitivity of muscle spindle endings, and reduces the amplitude of the stretch reflex (Gregory et al. 1987). In contrast, if the muscle length is held constant after the contraction, the stable cross bridges maintain intrafusal fibres taut and slack does not develop. Under these conditions, the background discharge of muscle spindle endings and their sensitivity is enhanced (Jahnke et al. 1989; Proske et al. 1992; Wilson et al. 1995), and the amplitude of the stretch reflex is high (Gregory et al. 1998). Furthermore, when a muscle contracts at short length and is then passively lengthened, the background discharge and sensitivity of the muscle spindle endings increase (e.g. Burke & Gandevia, 1992), as does amplitude of the stretch reflex (Wilson et al. 1999). Although alterations in thixotropy can occur in extrafusal as well as intrafusal muscle fibres, the changes in the stretch reflex evoked by conditioning voluntary contractions do not occur when contraction is produced by weak electrical stimulation of alpha motor axons (Gregory et al. 1998) and thus, are likely to reflect changes in intrafusal muscle fibres rather than changes in the extrafusal fibres or tendon.
Changes in the rate and pattern of spindle discharge that follow conditioning contractions may alter the excitability of spinal motoneurones. An H-reflex elicited once the muscle has been passively moved to an intermediate length is reduced after a conditioning contraction at a short length when compared with that elicited after a conditioning contraction at a long length (Wood et al. 1996; Gregory et al. 1998). That is, the change produced by conditioning voluntary contractions in the H-reflex is opposite to that in the stretch reflex. These H-reflex changes have been attributed to the central effects of the altered resting discharge of spindle endings (Gregory et al. 1990), although homosynaptic depression after the lengthening also contributes (Hultborn et al. 1996; Gregory et al. 1998).
Most neurones of the primate motor cortex receive afferent projections from the periphery: proprioceptive and tactile inputs reach the motor cortex (Hore et al. 1976; Lemon & Porter 1976; Zarzecki et al. 1978) via thalamo- cortical relays and cortico-cortical projections from the somatosensory cortex (Lemon, 1981; Huerta & Pons, 1990; for review Wiesendanger & Miles, 1982). Somatotopic projections to the cerebral cortex from low-threshold muscle afferents innervating upper limb muscles have been demonstrated in human subjects (Gandevia & Burke, 1988). In primates, the response of motor cortical neurones to proprioceptive and tactile inputs suggests their use in the execution and guidance of movements (e.g. Strick & Preston, 1978; for review, Phillips, 1986). In human subjects, alterations in the rate of firing of muscle spindle afferents can modify motor cortical excitability. Increases in firing rate of spindle afferents, induced by low-amplitude vibration applied over the muscle belly (Claus et al. 1988; Siggelkow et al. 1999), increase the size of the motor-evoked potential (MEP) elicited by transcranial magnetic stimulation. These changes were not accompanied by changes in the size of the response to transcranial electrical stimulation (Kossev et al. 1999). This suggests that the changes resulted from increased cortical rather than motoneuronal excitability.
This study aimed to determine if physiologically induced changes in the background discharge rate of spindle afferents alter cortical excitability. We used conditioning contractions to alter the firing of spindle afferents, and assessed the level of excitability of the motor pathways with transcranial magnetic stimulation, stimulation of the descending motor tracts and H-reflexes. The results suggest there is an increase in cortical excitability associated with an increased spindle afferent discharge.
Methods
From 27 volunteers, 11 subjects (5 female, 6 male) with no history of neuromuscular disease completed the study successfully. Subjects gave informed consent to the experimental procedures, which were approved by the local ethics committee (University of New South Wales) and were applied according to the Declaration of Helsinki. Subjects sat with the right shoulder and elbow flexed and the forearm resting on a table. The forearm was supported in the neutral position with the hand in an isometric myograph, which measured the force of wrist flexion (Fig. 1). The myograph was mounted on a rotating disk and the hand was positioned so that the axis of the wrist was aligned with that of the disk. Wrist flexion and extension could be altered by rotating the disk. Subjects performed a series of isometric wrist flexion contractions, designed to alter the thixotropic state of the wrist flexor muscles. Each contraction was followed by a test stimulus to elicit a stretch reflex, an H-reflex or an MEP in the wrist flexors. Surface electromyographic (EMG) recordings were made with electrodes (1 cm diameter) positioned over flexor carpi radialis (FCR) (Fig. 1). Prior to the main experiment, nine subjects in whom it was not possible to elicit a stable H-reflex in the electromyographically silent FCR and four subjects who were unable to relax reliably during testing were excluded from the study. Another three subjects were later excluded because the tendon tap response did not show evidence of thixotropy.
Figure 1. Experimental set-up.
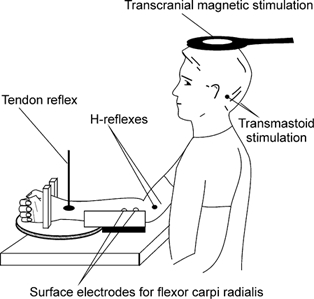
The hand was fixed in a myograph, which measured the force of wrist flexion. The myograph was mounted on a rotating disk over the joint axis, allowing wrist flexion and extension. Surface EMG recordings from the flexor carpi radialis allowed measurement of the response to each of the test stimuli (tendon tap, surface stimulation of the median nerve proximal to the elbow, transcranial magnetic stimulation, transmastoid electrical stimulation).
From the 11 successful volunteers, three completed a second series of experiments using the same recording and contraction protocols (described below). In this series, the test stimulus for the first set of contractions was a tendon tap. On the basis of a response to the tendon tap indicative of a thixotropic effect in the wrist flexors, subjects performed a second set of contractions for which the test stimulus was transcranial magnetic stimulation. If responses to the transcranial magnetic stimulation were different in the two conditions, and thus suggested a change in cortical excitability, subjects performed a third set of contractions for which the test stimulus was transmastoid electrical stimulation.
Recording protocol
Force of wrist flexion and EMG activity were sampled from 50 ms before to 100 ms after each test stimulus. Subjects received visual feedback of force, and auditory feedback at high gain of the EMG. EMG (bandpass 16-3000 Hz) was sampled at 5000 Hz. Data were stored for off-line analysis (1401 interface; Cambridge Electronic Design, Cambridge, UK).
Contraction protocol
Two muscle ‘conditioning’ protocols were used. In both protocols subjects performed a voluntary isometric contraction lasting 5 s at 20 % maximal voluntary contraction (MVC). Subjects then relaxed and the test stimulus was given 10 s after the end of the contraction. This interval should minimize any post-activation depression of the Ia synapse (Hultborn et al. 1996). Auditory feedback of EMG helped subjects to relax. Trials in which EMG activity persisted following the contraction were excluded from analysis (≈3 % of trials). In a ‘contract-long’ condition subjects contracted with the wrist held in 30 deg extension. To introduce slack into the intrafusal fibres the wrist was then passively returned to the neutral position immediately subjects relaxed. In a ‘contract-test’ condition subjects performed the contraction with the wrist in the neutral position and, to maintain stiffness in the intrafusal fibres, remained in this position until after the test stimulus. Test stimuli, described below, were always delivered with the wrist in the neutral position and FCR relaxed. Subjects rested for ≈10 s after the test stimulus before the next contraction. For statistical analysis each contract-long condition and its succeeding contract-test condition were considered as a pair.
Test stimuli
Each test stimulus was applied during a set of 15 consecutive pairs of contractions, with several minutes rest between sets.
Tendon reflexes were elicited using a tendon hammer applied to the tendon of the FCR just proximal to the wrist crease. There was no difference in the magnitude of the tendon taps in the contract-long and contract-test conditions as measured from change in the force record.
H-reflexes were elicited via surface electrodes over the median nerve just proximal to the elbow with the cathode proximal (pulse width 1 ms). Stimulus intensity and electrode position were adjusted to obtain a stable H-reflex at an amplitude of ≈50 % Hmax (maximal H-reflex). With this stimulus, M-waves were present in all but one subject, and remained the same in the contract-long and contract-test conditions.
Transcranial magnetic stimuli were delivered using a round coil (13.5 cm o.d. at 60-90 % maximal output, Magstim 200; Magstim Co., Dyfed, UK). The coil was centred close to the vertex and oriented to stimulate the left hemisphere preferentially. Small adjustments were made to the coil position to ensure that the stimuli evoked optimal motor-evoked potentials (MEP) in FCR.
Transmastoid electrical stimuli were delivered via electrodes fixed over the mastoid processes (100 μs pulse, 375-435 V, D180 stimulator; Digitimer). Such stimuli activate corticospinal axons at the cervicomedullary junction (Ugawa et al. 1991, Gandevia et al. 1999; Taylor et al. 2002). At high stimulation intensities direct activation of the motor roots is possible and is indicated by a latency shift in the response. The latency of the cervicomedullary motor-evoked potential (CMEP) was monitored and the intensity of the stimulus selected to stimulate the descending corticospinal tracts and not the motor roots.
Analysis and statistics
In all trials, peak-to-peak amplitude and area of the evoked responses were measured. As both measures showed parallel changes, only peak-to-peak amplitudes were used for statistical analysis.
In each subject differences in the response amplitude (tendon tap, H-reflex, MEP or CMEP) between consecutive pairs of contract-long and contract-test conditioning were assessed using Wilcoxon's signed-rank test. Three subjects showed no significant change in the response to tendon tap and were excluded from subsequent analysis. For group comparisons for the remaining subjects (n = 11), subjects’ mean response amplitudes for contract-long and contract-test conditioning were analysed with Student's paired t test. Significance was set at the 5 % level.
Results
Subjects performed 15 pairs of conditioning contractions designed to induce history-dependent changes in the discharge of muscle spindle afferents of wrist flexor muscles. In different sets of contractions, each contraction was followed by a test stimulus to elicit a tendon reflex, an H-reflex, an MEP or a CMEP. Despite continuous auditory feedback of EMG, subjects had more difficulty relaxing after the ‘contract-long’ than after the ‘contract-test’ conditioning contraction.
The key comparison was between the size of the test response after contract-long conditioning (muscle spindles slack) and its size after contract-test conditioning (muscle spindles not slack). After contract-long conditioning there was a reduction in the size of the tendon reflex, no change in the size of the H-reflex, and a small but significant reduction in the size of the MEP. The expected reduction in the tendon reflex following the contract-long conditioning contraction was taken as evidence of a thixotropic change in the muscle spindle endings. Responses in one subject to the tendon tap, H-reflex and MEP are shown in Fig. 2, with normalized data for all subjects in Fig. 4. Expressed as percentages of the maximal M-wave, the mean response evoked after contract-test conditioning was 25 ± 12.2 % (mean ± s.d.) for the tendon reflex, 19 ± 16.5 % for the H-reflex and 14 ± 8.9 % for the MEP.
Figure 2. Superimposed and average responses from a single subject.
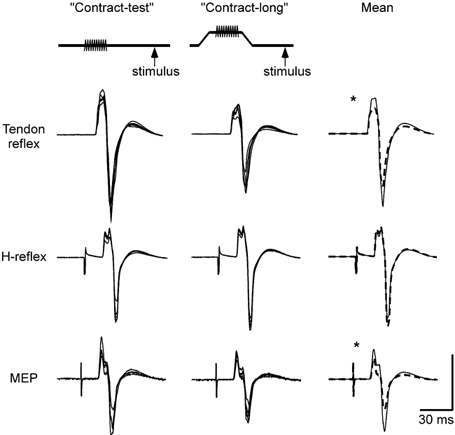
Schematic diagram at the top shows the ‘contract-test’ and ‘contract-long’ protocols (contraction indicated by the shaded line and timing of the test stimulus by an arrow). The top row of traces shows tendon reflexes. Left traces are 5 superimposed responses for the contract-test condition and the centre panel is 5 corresponding superimposed responses for the contract-long condition. Right traces show the mean of all responses in the two conditions (n = 15) for the contract-test responses (continuous line) and the contract-long responses (dashed line). Middle and bottom rows show the H-reflex and MEPs for the same subject. Vertical calibration: tendon reflex 2.5 mV, H-reflex 2.5 mV and MEP 1.0 mV. * Significant change (P < 0.05).
Figure 4. Normalized data for the group.
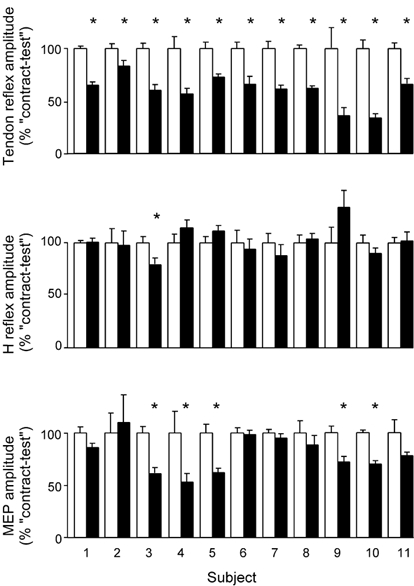
Data are shown for each subject following contract-test conditioning (□) and contract-long conditioning (▪), with data normalized to the average response from 15 trials following contract-test conditioning for each test stimulus (mean ± s.e.m.). In all subjects the size of the tendon reflex decreased after contract-long conditioning. One subject showed a significant change in the size of the H-reflex. In 5 subjects there was a small but significant change in the MEP (as indicated by the asterisks). All statistics were performed on non-normalized data with normalization conducted only for illustrative purposes.
Amplitude of the tendon reflex
After contract-long conditioning the amplitude of the tendon reflex was significantly reduced in the eleven subjects included in analysis by an average of 40 % (range 17-66 %) compared with after contract-test conditioning (Fig. 4). This reduction occurred in at least 13 of the 15 pairs of contractions in all subjects and in all pairs in three subjects (P < 0.05 in each subject, Wilcoxon's signed-rank test). For the group, the reduction in the tendon jerk was significant (P = 0.001, paired t test).
Amplitude of the H-reflex
The amplitude of the H-reflex changed significantly in only one subject following contract-long conditioning (Fig. 4). Of the remaining 10 subjects, some had a larger and some a smaller H-reflex compared to the contract-test condition with these non-significant changes varying from -12.5 % to +34 %. For the group, the average change in amplitude following contract-long conditioning was an increase of 1.4 %.
Amplitude of the MEP response
For the group, the amplitude of the MEP was significantly reduced by 20.5 % (P = 0.002) following contract-long conditioning. While one subject showed a (non-significant) 10 % increase in the MEP (Fig. 4), the MEP decreased after contract-long conditioning in the other 10 subjects with a maximal reduction of 47 %. In five subjects the reduction was statistically significant with a smaller MEP following contract-long than contract-test conditioning in 11 or more of the 15 pairs of contractions (P < 0.05 in each subject).
Amplitude of the CMEP response
Three subjects with changes in the tendon tap response consistent with thixotropic effects in the wrist flexors and who also showed significant changes in the MEP response repeated the contraction protocol with transmastoid electrical stimulation as the test stimulus (Fig. 3). In two of the subjects there was no significant change in the CMEP following contract-long conditioning but in one subject the CMEP was significantly smaller. In this subject, the MEP declined 37 % while the CMEP was decreased by 17 %.
Figure 3. Superimposed and average MEPs and CMEPs from a single subject.
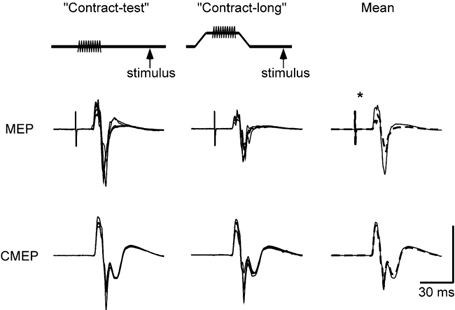
Responses from the subject shown in Fig. 2 recorded on a separate day. Left traces are 5 superimposed responses for the contract-test condition and the centre panel is 5 corresponding superimposed responses for the contract-long condition. Right traces show the mean of all responses in the two conditions (n = 15) for the contract-test responses (continuous line) and the contract-long responses (dashed line). Top row of traces shows MEPs and the bottom row responses to transmastoid stimulation (CMEPs). Vertical calibration: MEP 1.0 mV and CMEP 2.0 mV. * Significant change (P < 0.05).
Discussion
In these experiments we used conditioning voluntary contractions to alter the thixotropic properties of the wrist flexor muscles. As evidence of a ‘conditioning’ effect the size of the tendon tap response decreased following a conditioning voluntary contraction which was designed to introduce slack to the intrafusal fibres when compared to its size following a contraction designed to remove slack. With the same conditioning procedure the size of the motor-evoked potential to transcranial magnetic stimulation decreased without a corresponding change in the H-reflex.
The change in the size of the tendon tap response after conditioning contractions can be attributed to changes in the properties of the muscle spindle (and not the extrafusal fibres; Gregory et al. 1998). When there is slack in the intrafusal fibres, for example following contract-long conditioning, a portion of the mechanical deformation of an applied tendon tap must take up this slack before any stretch is applied to the intrafusal fibres. This results in a delay and a reduction in the magnitude of the stretch of the muscle spindle. The resulting afferent volley is therefore reduced in size (Jahnke et al. 1989; Gregory et al. 1990; Proske et al. 1992), and the amplitude of the evoked reflex is similarly reduced. In the absence of slack in the intrafusal fibres, for example following contract-test conditioning, a larger portion of the mechanical deformation of the tendon tap reaches the sensory ending. This results in a larger afferent volley and a larger tendon tap response. In addition to the increased responsiveness to stretch, the tonic firing of muscle spindle afferents is also increased after a voluntary contraction which reduces the slack in the intrafusal fibres (Wilson et al. 1995).
In the current study a larger MEP followed contract-test than contract-long conditioning. This suggests that changes in the background firing of spindle afferents, produced by a simple physiological manoeuvre, had a central effect and is consistent with a study in the rat in which passive repositioning of the forelimb altered the EMG responses to stimulation of the motor cortex (Sanes et al. 1992). The effects of the conditioning contractions on the motor pathway were weak. Overall, there was a 20 % change in size of the MEP and no consistent effect on the H-reflex. Changes in the size of the MEP can reflect changes in the level of excitability at the spinal motoneurone pool, at a premotoneuronal level in non-monosynaptic pathways to the motoneurone, or at the motor cortex (for review Rothwell et al. 1991; Pierrot-Deseilligny, 1996). In most subjects, it is unlikely that motoneuronal ‘excitability’ was altered at the chosen contraction-stimulus interval (10 s) as the H-reflex and the CMEP were little affected by conditioning contractions. In two subjects, changes did occur in the H-reflex or the CMEP, but, even in these subjects the change in the MEP was twice that in the H-reflex or CMEP, and so is consistent with an effect of the altered discharge of spindle afferents at a cortical or premotoneuronal level in addition to the action at the motoneurone pool.
The greater changes in the MEP than in the CMEP suggest that this effect did not occur at a subcortical level. The CMEP is elicited through stimulation of many of the same corticospinal axons as those which convey the descending volleys that evoke the MEP (Ugawa et al. 1991; Taylor et al. 2002). This stimulation occurs at the cervicomedullary junction, so that any actions of the spindle afferents on interneurones in a non-monosynaptic pathway to the motoneurone should affect the CMEP in the same way as the MEP. In addition, while alterations in transmission through a propriospinal-like system have been demonstrated at rest in human subjects, they are more common and robust with voluntary contraction (Burke et al. 1992). Higher intensities of transcranial magnetic stimulation, like those in the current study, are reported to inhibit propriospinal interneurones (Nicolas et al. 2001) so that alterations in spindle input are unlikely to have been effective in changing transmission of the descending volleys. Thus, we suggest that the most likely cause for the small but significant increase in the MEP after contract-test conditioning is an increase in excitability of motor cortical neurones.
Although our study showed no significant overall change in the H-reflex, previous authors found that the H-reflex was smaller after contractions designed to remove slack from intrafusal fibres than after contractions which introduce slack. These changes in the H-reflex occurred with contraction-stimulus intervals of 5 s but did not occur when the interval increased to 10 s (Wood et al. 1996; Gregory et al. 1998). Similarly, Hultborn and colleagues (1996) observed a depression of the H-reflex after passive stretching of a muscle. This depression probably resulted from a pre-synaptic post-activation depression of muscle spindle afferents activated by the muscle stretch and largely recovered within 10 s. Our contraction-stimulus interval of 10 s was chosen to minimize any post-activation depression (Hultborn et al. 1996, Gregory et al. 1998). Additionally, our conditioning procedures did not involve passive stretching of the wrist flexors, which produces an ‘initial burst’ of spindle afferent activity which prolongs the post-activation depression (Gregory et al. 1998). After ‘contract-long’ conditioning post-activation depression would be expected to lead to bigger H-reflexes if there were effectively less transmitter depletion, whereas the MEP was reduced after this procedure.
The present study provides evidence of the importance of physiologically relevant changes in the tonic input to the motor cortex from spindle afferents of resting muscle in determining the apparent level of cortical excitability. Responses to stimulation of the motor cortex increased after isometric voluntary contractions which increase the background discharge rate of spindle afferents. Hence, motor cortical excitability may change according to the naturally occurring variations in afferent input, and our results assign new significance to the role of muscle spindle afferents in contributing to this excitability.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (no. 3206). We are grateful to several colleagues for comments on the experimental procedures.
References
- Burke D, Gandevia SC. The human muscle spindle and its fusimotor control. In: Ferrell W, Proske U, editors. Neural Control of Movement. London: Plenum Press; 1992. pp. 19–25. [Google Scholar]
- Burke D, Gracies JM, Meunier S, Pierrot-Deseilligny E. Changes in presynaptic inhibition of afferents to propriospinal-like neurones in man during voluntary contractions. Journal of Physiology. 1992;449:673–687. doi: 10.1113/jphysiol.1992.sp019108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus D, Mills KR, Murray NM. Facilitation of muscle responses to magnetic brain stimulation by mechanical stimuli in man. Experimental Brain Research. 1988;71:273–278. doi: 10.1007/BF00247487. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Burke D. Projection to the cerebral cortex from proximal and distal muscle in the human upper limb. Brain. 1988;111:389–403. doi: 10.1093/brain/111.2.389. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Petersen N, Butler JE, Taylor JL. Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. Journal of Physiology. 1999;521:749–759. doi: 10.1111/j.1469-7793.1999.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JE, Mark RF, Morgan DL, Patak A, Polus B, Proske U. Effects of muscle history on the stretch reflex in cat and man. Journal of Physiology. 1990;424:93–107. doi: 10.1113/jphysiol.1990.sp018057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Changes in the size of the stretch reflex of cat and man attributed to aftereffects in muscle spindles. Journal of Neurophysiology. 1987;58:628–640. doi: 10.1152/jn.1987.58.3.628. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Wise AK, Wood SA, Prochazka A, Proske U. Muscle history fusimotor activity and the human stretch reflex. Journal of Physiology. 1998;513:927–934. doi: 10.1111/j.1469-7793.1998.927ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hore J, Preston JB, Cheney PD. Responses of cortical neurons (areas 3a and 4) to ramp stretch of hindlimb muscles in the baboon. Journal of Neurophysiology. 1976;39:484–500. doi: 10.1152/jn.1976.39.3.484. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Pons TP. Primary motor cortex receives input from area 3a in macaques. Brain Research. 1990;537:367–371. doi: 10.1016/0006-8993(90)90388-r. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Experimental Brain Research. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Jahnke MT, Proske U, Struppler A. Measurements of muscle stiffness, the electromyogram and activity in single muscle spindles of human flexor muscles following conditioning by passive stretch or contraction. Brain Research. 1989;493:103–112. doi: 10.1016/0006-8993(89)91004-4. [DOI] [PubMed] [Google Scholar]
- Kossev A, Siggelkow S, Schubert M, Wohlfarth K, Dengler R. Muscle vibration: different effects on transcranial magnetic and electrical stimulation. Muscle and Nerve. 1999;22:946–948. doi: 10.1002/(sici)1097-4598(199907)22:7<946::aid-mus22>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Functional properties of monkey motor cortex neurones receiving afferent input from the hand and fingers. Journal of Physiology. 1981;311:497–519. doi: 10.1113/jphysiol.1981.sp013601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN, Porter R. Afferent input to movement-related precentral neurones in conscious monkeys. Proceedings of the Royal Society. 1976;B 194:313–339. doi: 10.1098/rspb.1976.0082. [DOI] [PubMed] [Google Scholar]
- Nicolas G, Marchand-Pauvert V, Burke D, Pierrot-Deseilligny E. Corticospinal excitation of presumed cervical propriospinal neurones and its reversal to inhibition in humans. Journal of Physiology. 2001;533:903–919. doi: 10.1111/j.1469-7793.2001.t01-1-00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CG. Movements of the Hand. XVII. Liverpool: Liverpool University Press; 1986. [Google Scholar]
- Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical propriospinal premotoneurons. Progress in Neurobiology. 1996;48:489–517. doi: 10.1016/0301-0082(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL, Gregory JE. Muscle history dependence of responses to stretch of primary and secondary endings of cat soleus muscle spindles. Journal of Physiology. 1992;445:81–95. doi: 10.1113/jphysiol.1992.sp018913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles: a review. Progress in Neurobiology. 1993;41:705–721. doi: 10.1016/0301-0082(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Experimental Physiology. 1991;76:159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Wang J, Donoghue JP. Immediate and delayed changes of rat motor cortical output representation with new forelimb configurations. Cerebral Cortex. 1992;2:141–152. doi: 10.1093/cercor/2.2.141. [DOI] [PubMed] [Google Scholar]
- Siggelkow S, Kossev A, Schubert M, Kappels H-H, Wohl W, Dengler R. Modulation of motor evoked potentials by muscle vibration: the role of vibration frequency. Muscle and Nerve. 1999;22:1544–1548. doi: 10.1002/(sici)1097-4598(199911)22:11<1544::aid-mus9>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Strick PL, Preston JB. Sorting of somatosensory afferent information in primate motor cortex. Brain Research. 1978;156:364–368. doi: 10.1016/0006-8993(78)90520-6. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Petersen NT, Butler JE, Gandevia SC. Interaction of transcranial magnetic stimulation and electrical transmastoid stimulation in human subjects. Journal of Physiology. 2002;541:949–958. doi: 10.1113/jphysiol.2002.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Annals of Neurology. 1991;29:418–427. doi: 10.1002/ana.410290413. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M, Miles TS. Ascending pathway of low-threshold muscle afferents to the cerebral cortex and its possible role in motor control. Physiological Reviews. 1982;62:1234–1270. doi: 10.1152/physrev.1982.62.4.1234. [DOI] [PubMed] [Google Scholar]
- Wilson LR, Gandevia SC, Burke D. Increased resting discharge of human spindle afferents following voluntary contractions. Journal of Physiology. 1995;488:833–840. doi: 10.1113/jphysiol.1995.sp021015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LR, Gracies J-M, Burke D, Gandevia SC. Evidence for fusimotor drive in stroke patients based on muscle spindle thixotropy. Neuroscience Letters. 1999;264:109–112. doi: 10.1016/s0304-3940(99)00181-0. [DOI] [PubMed] [Google Scholar]
- Wood SA, Gregory JE, Proske U. The influence of muscle spindle discharge on the human H reflex and the monosynaptic reflex in the cat. Journal of Physiology. 1996;497:279–290. doi: 10.1113/jphysiol.1996.sp021767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzecki P, Shinoda Y, Asanuma H. Projection from area 3a to the motor cortex by neurons activated from group I muscle afferents. Experimental Brain Research. 1978;33:269–282. doi: 10.1007/BF00238065. [DOI] [PubMed] [Google Scholar]


