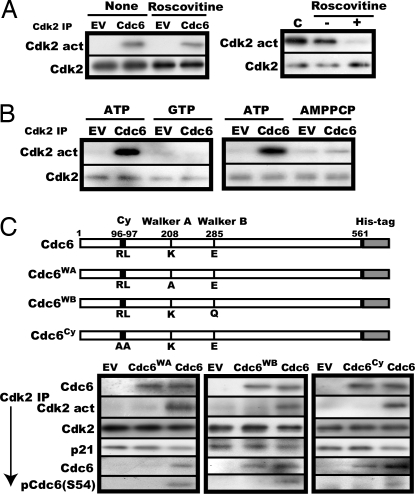
Fig. 3.
Both ATPase and cyclin-binding motifs in Cdc6 are essential for activation of p21-associated Cdk2 in vitro. (A) (Left) The same activation assay was performed as in Fig. 2B with the addition of 100 μM roscovitine followed by removal of roscovitine and assay of the activity and amount of Cdk2. (Right) Active Cdk2 immunoprecipitated from a logarithmically growing NRK cell lysate was incubated with 100 μM roscovitine under the same condition as for the Cdk2 activation assay, washed (−) or unwashed (+), and assayed for the activity and amount of Cdk2. C stands for direct assay of the Cdk2 without incubation with roscovitine. (B) The same activation as in Fig. 2B was performed with 10 mM ATP, GTP, or AMPPCP. (C) Immunoprecipitated p21-bound Cdk2 was incubated in the activation reaction mixture with 10 μl of purified Cdc6, Cdc6WA, Cdc6WB, or Cdc6Cy, whose structures are illustrated, and determined for the activity and the amount of Cdk2 and coimmunoprecipitated p21, Cdc6, and Ser-54-phosphorylated Cdc6. In parallel, the relative amounts of the Cdc6s added to the activation reaction were compared by immunoblot.