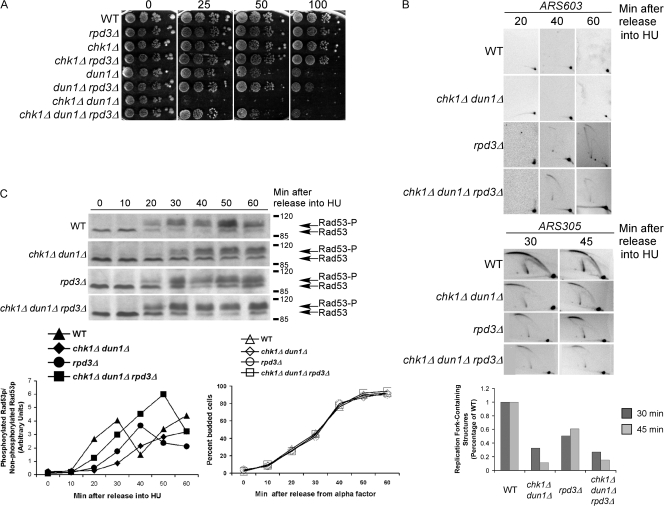
Figure 3.
Deletion of RPD3, which encodes a histone deacetylase, suppresses the HU sensitivity of chk1Δ dun1Δ cells. (A) Cells were spotted onto YPD medium containing the indicated concentration of HU (mM). (B) Analyses of replication fork–containing structures were performed as in Fig. 1 B, using a probe for the late chromosomal origin ARS603 (top) or the early chromosomal origin ARS305 (bottom). (C, top)Rad53p phosphorylation in response to HU treatment is restored to wild-type levels and kinetics in chk1D dun1D cells by additional replication origins that are activated in early S phase. Rad53p phosphorylation was monitored as in Fig. 1 D, except that the cells were released at 18°C. (C, bottom left) Ratio of phosphorylated to nonphosphorylated Rad53p. (C, bottom right) Budding index.