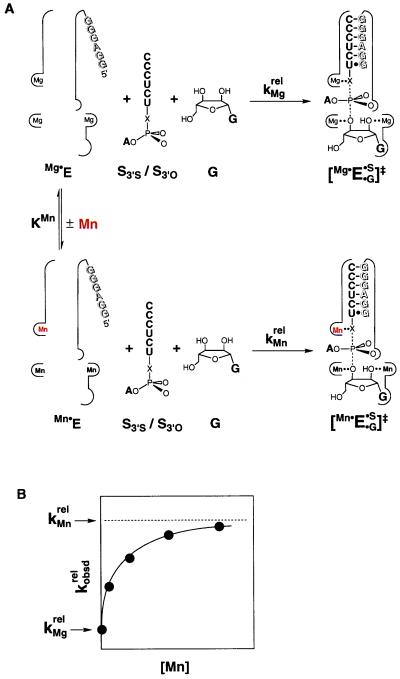
Figure 3.
Schematic description of the method for determining the affinities of rescuing metal ions. (A) Equilibrium for binding of Mn2+ (red) to metal site A in the free ribozyme (KMn), and reaction of S3′S and S3′O with Mg2+ and Mn2+ bound at site A, with relative rate constants of kMgrel and kMnrel, respectively (krel = kS3′S/kS3′O). The ribozyme active site is schematically represented by the thin outline, with the IGS of the ribozyme shown by shaded letters. Addition of Mn2+ would lead to partial occupancy of all metal ion sites, depending on the metal ion concentration and the Mn2+ affinity of each site. This is represented by the Mn2+ occupancy of metal sites B and C in the Mn⋅E and [Mn⋅E⋅G⋅S]‡ species. (B) The Mn2+ concentration dependence of the observed reactivity of S3′S relative to S3′O (kobsdrel) predicted from the model of A. Analysis of the Mn2+ concentration dependence gives the Mn2+ affinity for metal site A in the free ribozyme, as described in the text.