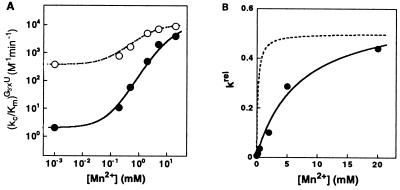
Figure 6.
Mn2+ rescues the reactivity of G3′SU. (A) Effect of Mn2+ on the rate of the reverse reaction E⋅P + G3′XU → E⋅S + G3′XH [(kc/Km)G3′XU] with G3′SU (●) and G3′OU (○), determined as described in Materials and Methods. The effect of Mn2+ on the reaction of G3′OU arises from a Mn2+ distinct from the Mn2+ ions investigated herein (unpublished results). (B) The effect of Mn2+ on the reactivity of G3′SU relative to G3′OU [krel = (kc/Km)G3′SU/(kc/Km)G3′OU]. The solid line is a fit of the data to Eq. 3b, derived from the model of Fig. 3A, and gives KMnB = 7 ± 1 mM. The dashed line is a hypothetical Mn2+ concentration dependence of krel expected if MnC2+, which binds to E⋅P with a dissociation constant of 0.19 mM (7), were responsible for rescue of G3′SU.