Abstract
Biogenic amines, such as serotonin and dopamine, can be important in reinforcing associative learning. This function is evident as changes in memory performance with manipulation of either of these signals. In the insects, evidence begins to argue for a common role of dopamine in negatively reinforced memory. In contrast, the role of the serotonergic system in reinforcing insect associative learning is either unclear or controversial. We investigated the role of both of these signals in operant place learning in Drosophila. By genetically altering serotonin and dopamine levels, manipulating the neurons that make serotonin and dopamine, and pharmacological treatments we provide clear evidence that serotonin, but not dopamine, is necessary for place memory. Thus, serotonin can be critical for memory formation in an insect, and dopamine is not a universal negatively reinforcing signal.
Keywords: biogenic amines, dopamine, learning, white-ABC transporter, reinforcement
The neural systems containing biogenic amines, such as dopamine and serotonin, may mediate reinforcement information to influence memory performance. In the monkey for example, activity in the dopaminergic system is modulated based on expected reward (1), and the phasic output of these neurons may regulate memory performance (1, 2). In some invertebrates the biogenic amines have also been shown to be critical for conditioning (3–5). Within the insects, however, dopamine is the only biogenic amine clearly implicated in negatively reinforced associative memory (6–8). Indeed, and interestingly, dopaminergic system activation can be a sufficient reinforcing signal for olfactory conditioning in Drosophila larvae (9). Thus, support grows for a general function of the dopaminergic system in negatively reinforced memory. Whether serotonin has a role in insect learning is less clear (10), and in Drosophila it is controversial (11–13). Here, we investigated the influence of serotonin and dopamine on reinforcement of place learning in Drosophila.
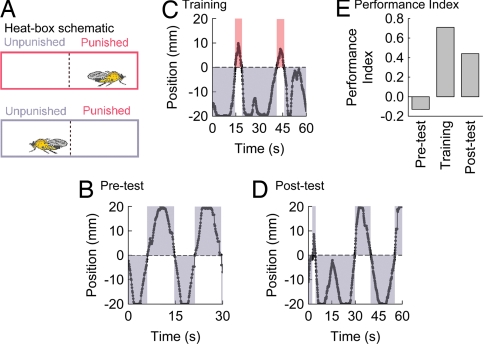
The “heat box” can be used to rapidly condition place memories in Drosophila (14, 15). In this paradigm, single flies are allowed to wander in a chamber that is lined top and bottom with Peltier heating elements (Fig. 1) (16, 17). A series of light sensors on one side of the chamber tracks the behavior of a fly, and when the animal moves to a predetermined half, the whole chamber heats to a nonpreferred (aversive) temperature. With experience, normal flies avoid the chamber-half associated with rising temperatures (15, 16, 18). A test performed after conditioning, when the danger of rising temperature is removed, is used to measure place memory. Importantly, one can dissociate acquisition from reinforcement processing defects by the performance of mutant flies after short and long training sessions (19). Flies that are mutant for a type-1 adenylyl cyclase (i.e., rutabaga) show poor memory performance after short periods of conditioning but normal memory after longer training, emphasizing the memory acquisition function for this protein. In contrast, flies mutant for the white-ABC transporter have low memory performance after both short and long training sessions, similar to normal flies' memory performance with lower-temperature reinforcement (19). This finding suggests the white-ABC transporter provides a function critical for reinforcement processing.
Fig. 1.
Schematic of heat-box and sample position traces/performance indices. (A) Individual flies walk in a small chamber, lined top and bottom with heating elements. During a training session, when a fly moves to one half of the chamber the whole chamber heats to a defined temperature (the midline is represented by a dashed line, heating is represented as a red box). When a fly returns to the previous chamber half the chamber cools (represented in blue). The behavior of an individual fly is represented from a short period from each of the pretest, training, and posttest phases. (B) In the pretest, flies typically walk from chamber end to end, spending about equal time in each chamber half (the amount of blue shading is similar above and below the midline). (C) In the training phase, flies will walk past the midline and the temperature in the chamber rises (red shading). Normal flies spend less time on the side of the chamber associated with high-temperature punishment during training (compare red and blue shading). (D) In a memory posttest, flies continue to avoid the side formerly associated with high temperature (more blue shading on the bottom half of the position trace). (E) Side preference behavior is quantified in a performance index, where time spent on the high-temperature side is subtracted from the time on the low-temperature side, all divided by the total time within a session. The behavior of the fly in B–D is quantified here and follows the form typically found when averaged across many flies. The performance index can range from 1 to −1. Absolute avoidance of the high-temperature-associated chamber half gives a value of 1. A value of zero indicates no side preference.
After the initial observation of reinforcement processing deficits in white-ABC transporter mutant flies, in this study we investigated whether serotonin and/or dopamine are critical for this function. We followed this hypothesis because the white-ABC transporter is known to be critical in the translocation of tryptophan and guanine into at least some tissues (20–22). Tryptophan is converted in two enzymatic steps to serotonin. Furthermore, guanine is a precursor to tetrahydropterin, an obligate cosubstrate for the enzymes that catalyze the synthesis of serotonin and dopamine (23–25). We measured levels of serotonin, dopamine, and a third biogenic amine octopamine in wild-type and white mutant fly head tissue. Furthermore, using spatially restricted transgenic expression of RNAi-white and the tetanus toxin light chain (TeTxLC), we examined the behavioral function of the serotonergic and dopaminergic neural systems. Pharmacological manipulation of serotonin and dopamine levels and measurement of behavioral consequences completed these investigations. The results of these experiments indicate that serotonin is necessary for high-temperature negatively reinforced place memory but dopamine is not. Therefore, serotonin can be critical for associative learning in the insects and dopamine is not a universal negative reinforcing cue.
Results
Altered Biogenic Amines in White Mutant Flies.
Flies mutant for the white-ABC transporter (w1118) have memory performance levels that are ≈60% of wild-type levels in the heat box (Table 1), similar to levels found in wild-type CS flies that are conditioned with lower temperatures (19). And, the white-ABC transporter-dependent memory deficit has been dissociated from changes in the ability to sense and avoid a high-temperature source (>41°C) in tests for thermosensitivity (19). To test the notion that the white-ABC transporter provides critical substrates for biogenic amine synthesis, we measured the levels of serotonin, dopamine, and octopamine in white mutant flies. We included tests for octopamine as it has been implicated in learning in both the honey bee and Drosophila (7, 10, 26). We found that white mutant flies have ≈30% of wild-type levels of serotonin and dopamine (Table 1). Although the octopamine level in white mutant flies is ≈80% of normal, this reduction does not reach significance (Table 1). Thus, low serotonin and dopamine levels are correlated with abnormal place conditioning.
Table 1.
Wild-type CS and white mutant flies' memory and biogenic amine levels
| Genotype | Memory, PIN = 221 | Serotonin, pg/headN = 16 | Dopamine, pg/headN = 10 | Octopamine, pg/headN = 8 |
|---|---|---|---|---|
| CS | 0.78 ± 0.05 | 169.1 ± 36.3 | 598.8 ± 135.5 | 263.7 ± 60.7 |
| w1118 | 0.48 ± 0.06*** | 32.6 ± 9.2** | 147.8 ± 31.4* | 232.7 ± 91.1 |
Memory was tested after 20 min of training. Biogenic amines were quantified from head extracts. Measurements from wild-type CS and w1118flies in a CS genetic background were recorded in parallel. Values are means ± SEMs. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Transgenic Manipulation of Serotonergic/Dopaminergic Systems.
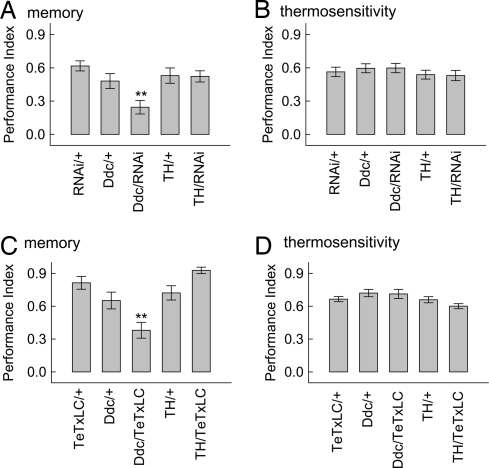
Additional support for the role of serotonin and/or dopamine in regulating memory in Drosophila can be gained from manipulating the function of the serotonergic and dopaminergic neural systems. Furthermore, one might discriminate between the function of dopamine and serotonin in place conditioning by using two different GAL4 drivers. The dopa decarboxylase (Ddc)-GAL4 driver is expressed in a restricted set of neurons that includes the serotonergic and dopaminergic neurons (ref. 27 and see below). The tyrosine hydroxylase (TH)-GAL4 driver is expressed in the dopaminergic neurons (28). We first addressed the role of the White-ABC transporter by expressing an RNAi transgene under UASGAL4 control in these neurons [supporting information (SI) Fig. S1] (29). Only flies expressing the white-ABC transporter-RNAi transgene under Ddc control showed conditioned memory deficits compared with all genetic controls (Fig. 2). The TH-GAL4 driven expression of the white-ABC transporter-RNAi transgene had no effect on conditioned behavior. In all genetic manipulations there were no significant differences in control experiments testing the ability of flies to sense and avoid a 41°C temperature source, the same temperature used for conditioning (Fig. 2). The effect of down-regulating white-ABC transporter expression on place memory likely reflects an endogenous function for the white-ABC transporter as white-mRNA has been detected in head tissue lacking any of the visual systems (30) and a white promoter driving expression of a marker protein (31) can be colocalized with serotonin expression (Fig. S1).
Fig. 2.
The Ddc-positive neurons are necessary for normal memory performance. (A) When the UASGAL4-RNAi-white transgene was expressed with Ddc-GAL4 driver, flies' memory performance was strongly reduced compared with all control genotypes [H (4, n = 643) = 28.3, P < 0.0001]. This finding was in contrast to TH-GAL4-driven UASGAL4-RNAi-white expression, where no deficits were found. Significant differences after multiple comparisons are presented, Ddc-GAL4/UASGAL4-RNAi-white with UASGAL4-RNAi-white/+ and Ddc/+ (**, P < 0.01). (B) Tests for the ability of flies with different genotypes to sense and avoid a 41°C temperature source did not find significant differences between genotypes [H (4, n = 382) = 3.37, P = 0.50]. (C) Blocking synaptic transmission by expressing the TeTxLC reveals a necessary role of the Ddc-positive neurons in memory formation. Flies with TeTxLC expression in the Ddc-positive and TH-positive neurons, and genetic controls, were trained in the heat box and tested for memory. Only flies expressing TeTxLC with the Ddc-GAL4 driver had a deficit in conditioned memory performance [H (4, n = 459) = 50.5, P < 0.0001, multiple comparisons indicate significant differences between Ddc-GAL4/UASGAL4-TeTxLC and both Ddc-GAL4/+ and UASGAL4-TeTxLC/+ performances (**, P < 0.01)]. (D) Tests for the ability to sense and avoid a 41°C high-temperature source in the thermosensitivity assay found a difference between these genotypes [H (4, n = 359) = 13.9, P < 0.01], which was caused by a difference between TH-GAL4/UASGAL4-TeTxLC and Ddc-GAL4/UASGAL4-TeTxLC flies (P < 0.05). The values represent means, and error bars are SEMs.
We next expressed the TeTxLC (UASGAL4-TeTxLC) in the Ddc- and TH-positive neurons to block synaptic transmission (32, 33) and tested these flies for memory formation. We reasoned that the TeTxLC effector transgene might reveal further functions of Ddc- or TH-positive neurons because cleaving synaptobrevin and blocking synaptic transmission might be a more drastic change in neuronal physiology than altering ABC transporter function. Expression of TeTxLC in the Ddc-positive neurons led to defects in memory, but left thermosensitivity unchanged (Fig. 2). Expression of TeTxLC in the TH-positive neurons had neither an effect on memory performance nor an effect on the avoidance of a 41°C source. Thus, the Ddc-GAL4 driver defines a neuronal set that requires synaptic transmission for proper memory formation. Furthermore, the RNAi-white transgene reduces Ddc-GAL4 neuron function to similar levels. Synaptic transmission from TH-GAL4-positive neurons is not necessary for heat-box conditioning. The results from experiments using two effector transgenes increases support for a role of the serotonergic system in place memory formation.
Manipulation of the Serotonergic System.
In an attempt to address the serotonergic system more directly, we used a TH-promoter GAL80 transgene (T.K. and Junko Kasuya, unpublished results). The GAL80 transcription repressor can effectively limit the effect of GAL4-dependent expression in the fly (34). We reasoned that as the TH promoter can effectively mark the dopaminergic neurons (28), suppressing GAL4 function with this promoter by GAL80 expression in combination with the Ddc-GAL4 driver would allow for more specific serotonergic system manipulation.
As a first step toward this goal, we identified the serotonergic neurons in the fly brain by using a monoclonal antibody against serotonin. We found between 38 and 41 serotonergic neurons per hemisphere (Table 2, Fig. 3, and Movie S1). Some of these neurons have been previously identified, including the lp2, se1, se2, and se3 neurons (35). We further identified neurons we term the serotonergic neurons of the anterior lateral protocerebrum (alp), anterior medial protocerebrum (amp), posterior medial protocerebrum (pmp), and posterior lateral protocerebrum (plp). Four other groups of serotonergic neurons have been previously described in the anterior part of the fly brain (35). Because we do not find serotonergic neurons in a similar position and examination of the images from ref. 35 shows structures consistent with the posterior part of the brain (e.g., mushroom body calyces and esophagus), it is likely the previously described “anterior” serotonergic neurons correspond to the pmp neurons shown here.
Table 2.
Location and number of serotonergic neurons
| Group | Cell body no./hemisphere | No. of Ddc-GAL4 + cells | Location |
|---|---|---|---|
| alp | 3 | 3 | Anterior cell body rind, lateral to midline |
| amp | 1 | 1 | Single cell body (≈10 μm diameter) lateral to antennal lobe, dorsal to ammc |
| lp2 | 9–11 | 3–4 | Cells between medulla/central neuropil |
| pmp | 13–14 | 8–10 | Posterior cell body rind, medial to the calyx, running dorso-ventral |
| plp | 3 | 3 | Posterior, between medulla/central neuropil |
| se1 | 3 | 2 | Anterior subesophageal neurons |
| se2 | 3 | 3 | Posterior to se1, lateral to midline |
| se3 | 3 | 3 | Posterior to se1, at the midline |
Fig. 3.
Serotonin in the adult Drosophila brain. (A) In this slightly oblique frontal image, depth is encoded from anterior to posterior as blue to red in 100 steps (0–125 relative units). Serotonergic neuron clusters are labeled (see Table 2 for description of each cluster and explanation of abbreviations). Also evident are innervations of the serotonergic neurons in the fan-shaped body (fb) and elsewhere. In B and C, confocal image sections including the fan-shaped body (fb) and rostral were used to generate the anterior images on the left. Different brains were imaged from the posterior to provide the caudal information. (B) Ddc-GAL4 drives expression of UASGAL4-GFP expression in a subset of the serotonergic neurons and two groups of nonserotonergic cells (described in Results). The anterior labeling has Ddc-GAL4/serotonin coexpression in a subset of the lp2, amp, alp (not shown), se1, se2, and se3 cells. Posterior Ddc-GAL4 / serotonin coexpression is detected in a subset of the pmp and plp cells. (C) TH-GAL4 drives UASGAL4-GFP expression in a distributed set of cells that do not overlap in expression with the serotonergic neurons. The white colocalization signal in the anterior medial ventral brain is an artifact of flattening these optical sections. (D) TH-GAL80 can effectively suppress TH-GAL4-driven UASGAL4-GFP expression. (Scale bar in A: 50 μm; applies to all.)
Next, we characterized the Ddc-GAL4 and TH-GAL4 drivers' expression with respect to the serotonergic system. We find that Ddc-GAL4 drives expression of UASGAL4-GFP in ≈220 neurons per hemisphere (excluding the optic lobes). We define three broad classes of Ddc-GAL4-positive neurons: (i) serotonergic neurons, (ii) distributed nonserotonergic neurons, and (iii) a cluster of nonserotonergic neurons in the anterior superior medial part of the cell body rind. First, the Ddc-GAL4-positive serotonergic neurons number between 26 and 29 (Table 2 and Fig. 3). Only a fraction of the lp2 and pmp neurons do not overlap with the Ddc-GAL4-positive neurons. Second, the ≈100 Ddc-GAL4-positive distributed nonserotonergic neurons, presumably including the dopaminergic neurons, are found in several regions of the cellular rind. Third, ≈100 nonserotonergic Ddc-GAL4-postive cell bodies are found in a cluster in the frontal cell rind. A predominant component of these cells includes what appears to be extrinsic mushroom body neurons. Examining TH-GAL4 driven UASGAL4-GFP expression we found ≈75 neurons per hemisphere, none of which overlap with the serotonergic signal (Fig. 3). The two white colocalization signals seen in Fig. 3 are an artifact of flattening these confocal images.
The effectiveness of the TH-GAL80 transgene in repressing expression in TH-GAL4-positive neurons was examined. We found that the TH-GAL4-driven UASGAL4-GFP expression could be completely blocked by the TH-GAL80 repressor element (Fig. 3). Furthermore, addition of TH-GAL80 to the Ddc-GAL4 driver reduced expression in the distributed nonserotonergic neuron population but did not appreciably alter the Ddc-GAL4-positive serotonergic neurons or the frontal cell body rind neurons (data not shown). Thus, the combination of Ddc-GAL4 and TH-GAL80 can be used to drive expression in nearly all of the serotonergic neurons, ≈100 neurons that innervate the mushroom bodies, and a handful of additional neurons distributed in the brain.
We next used the Ddc-GAL4;TH-GAL80 driver combination to manipulate synaptic transmission. Flies that expressed the UASGAL4-TeTxLC with the Ddc-GAL4; TH-GAL80 driver combination had a strongly reduced memory level compared with genetic controls (Fig. 4). Neither of these manipulations altered the ability of these flies to sense and avoid the high temperatures used as a negative reinforcer (Fig. 4). Thus, the serotonergic system can be manipulated independently of alteration of the dopaminergic system, which can alter place memory formation.
Fig. 4.
Manipulating the serotonergic system reduces memory performance. (A) Flies in which the TeTxLC was expressed in a set of CNS neurons, including the serotonergic neurons, showed a strong reduction in place memory [H (2, n = 286) = 14.5, P = 0.0007, multiple comparisons indicate significant differences between Ddc-GAL4;TH-GAL80/UASGAL4-TeTxLC and both Ddc-GAL4;TH-GAL80/+ and UASGAL4-TeTxLC/+ performances (*, P < 0.05)]. (B) Tests for the ability to sense and avoid a 41°C high-temperature source in the thermosensitivity assay found no significant differences between the genotypes tested [H (2, n = 210) = 1.20, P = 0.55]. (C) Adult flies fed am-W, reducing serotonin levels (36), had a significant difference in memory performance compared with flies fed on the media alone (sham) and flies fed am-Y, altering dopamine levels [H (2, n = 316) = 10.9, P = 0.004; multiple comparisons indicate significant differences between am-W-treated and both am-Y- and sham-treated flies (*, P < 0.05)]. (D) Tests for the ability to sense and avoid a 41°C high-temperature source in the thermosensitivity assay found no significant differences between the treatment groups (U test: Z = 0.77, P = 0.44, n = 190). Flies fed am-Y were not tested (ND) in the thermosensitivity assay. The values represent means, and error bars are SEMs.
Pharmacological Manipulation of Serotonergic/Dopaminergic Systems.
Pharmacological manipulation of serotonin and dopamine provides a third means of testing the role of these systems in Drosophila place memory formation. We fed flies the drug α-methyl tryptophan (am-W) to inhibit the synthesis of serotonin (36). Alternatively, flies were fed α-methyl tyrosine (am-Y) to inhibit TH activity and dopamine biosynthesis (37). Flies fed am-W, but not am-Y, for 2 days as adult animals had memory performance deficits (Fig. 4). We measured dopamine levels in fly heads to test the effectiveness of our am-Y feeding protocol. Flies fed am-Y had strongly reduced dopamine levels [am-Y: 29.9 ± 2.7, sham: 507.4 ± 109.5 pg dopamine per head; F(1,10) = 19.0, P = 0.001; n = 6 for each group]. Thus, our am-Y feeding reduces dopamine levels to 6% of normal, which does not alter memory performance. Also noted here is a reduced memory score in sham- and am-Y-treated flies compared with wild-type fly performance with a similar conditioning protocol but fed on cornmeal media. These differences could be caused by variation in flies' performance over time or to effects of the sham food. Regardless of this somewhat reduced memory background, feeding am-W to adult flies has a dramatic effect on memory performance levels. Finally, feeding flies am-W had no effect on temperature avoidance behavior (Fig. 4). These results support the conclusion that serotonin, but not dopamine, is necessary for place memory formation.
Discussion
Our key finding is that the serotonergic system is necessary for high temperature reinforced heat-box place learning. This conclusion is supported by three independent lines of evidence. First, white mutant flies have drastically reduced memory performance and show severely decreased levels of both serotonin and dopamine. Second, this mutant behavioral phenotype can be phenocopied by decreasing white expression in the cells that make serotonin. Furthermore, blocking synaptic transmission in the serotonergic cells by using the UASGAL4-TeTxLC transgene also reduces memory performance. Third, pharmacological reduction of serotonin reduces memory performance. Importantly, this effect can be seen after 2 days of drug feeding. This time frame indicates that the serotonergic system can function in the adult animal to influence memory independent of potential developmental effects. Although each of these manipulations may alter more than the serotonergic system, the common alteration with the three independent manipulations is a reduction in serotonergic function. Thus, the serotonergic neurons can be used in Drosophila as a negative reinforcing system. Whether the serotonergic reinforcement function is general will require additional tests of negatively and positively reinforced memory. Using manipulations that show the dopaminergic system is necessary for negatively reinforced olfactory learning (7, 9) we find no evidence for the role of dopamine in place learning, which indicates dopamine is not a general negative reinforcing system in the insects.
Memory strength, influenced by the intensity/amount of the reinforcer and measured for example as the rate or duration of conditioned behavior, is a common memory phenomenon (38). We use the general term “reinforcement processing” to address the mechanisms of memory strength/reinforcement intensity matching. The matching mechanism is also sometimes termed reinforcement learning (39). Biogenic amine systems appear to be critical for reinforcement processing in quite different organisms. In humans and monkeys, the performance level of a learned behavior can be quickly modulated by the intensity of a reinforcer, which is thought to be influenced by the dopaminergic system (2, 40, 41). Serotonin and dopamine also have a reinforcement function in the rodents, Aplysia, and Caenorhabditis elegans (3, 4, 42–45). That Drosophila use dopamine and serotonin for reinforcement argues that these neural systems have a conserved role in reinforcement processing.
Our findings that serotonin and dopamine are strongly reduced in white mutant flies have additional implications for Drosophila behavioral neuroscience. The white gene is extensively used as a genetic and transgenic marker in manipulating gene function, while its own influence may be overlooked. Only a few examples have previously identified a nonvisual, but nevertheless behaviorally significant, function to the white-ABC transporter. These include white overexpression effects on courtship behavior (46, 47) and loss-of-function effects on anesthesia resistance (30), aggression (48), and processing of low temperatures and electric shock (19). Whether the function of the white gene, and the effect of lower serotonin and dopamine, is restricted to these behaviors and place learning is open. One should not expect, however, genetic “marker” effects to be restricted to Drosophila. For example, different wild-type mouse strains used in generating transgenic animals have been shown to be quite different both in neurophysiological and behavioral learning tests (49). Whether these differences stem from a single gene is obviously not clear. Nevertheless, these studies highlight the unexpected and potentially strong influence on behavior of mutations that are used to facilitate molecular genetic manipulation.
Finally, that the white-ABC transporter influences serotonin/dopamine levels and memory performance may provide information on the function of homologous vertebrate genes. The white-ABC transporter is a member of the ABCG class of half-sized ABC transporters (50). The expression of some of the ABCG transporters has been identified in mouse brain (51, 52). And, interestingly, a mutation in ABCG1 has been found associated with some male depression patients (53). Thus, this class of transporters may be important for the regulation of biogenic amines in critical regions of the nervous system to influence behavior in multiple species. Importantly, we can take advantage of the relatively simple fly brain to close the gap between the molecular and circuit properties of associative learning and a conceptual model of operant behavior and learning (19, 54) to eventually provide for an integrated model of brain function.
Materials and Methods
Genetic Manipulations and Culture Conditions.
All flies were raised on our standard cornmeal-based fly food at 25°C, 60% relative humidity, in a 12:12 h light/dark cycle, unless otherwise noted. Standard genetic crosses were used to generate all experimental groups. All transgenic lines were outcrossed to our “cantonized” white mutant line, wCS13 (19), for at least six generations. The UASGAL4-RNAi-white (29) and UASGAL4-TeTxLC (CYO34–1) lines (55) additionally had their first chromosomes replaced with a wild-type version. The Ddc-GAL4 line is X-linked (27), and so all experimental and control animals were females. Crosses to generate Ddc-GAL4/+;UASGAL4-TeTxLC were performed at 18°C, and adult flies were transferred to 25°C 1 day before experimentation. A third chromosome insertion line of TH-GAL4 was used (28). A UASGAL4-nlsGFP line was used to detect GFP expression. Experimental animals used for behavioral studies were 2–5 days old and never anesthetized.
Pharmacological Treatments.
Two to 4-day-old wild-type CS flies were exposed to drugs in 1% agarose and 1% sucrose for 48 h. The sham control flies were in identical vials except without drug.
The drugs am-Y (2 mM) and am-W (20 mM) were mixed with cooling but melted agarose/sucrose solution and allowed to harden. Food coloring was added to the solution to allow for examination of feeding behavior with the drugs. The drugs did not seem to negatively affect feeding based on colored abdomens in flies exposed to the food for 2 days.
Biogenic Amine Measurement.
Serotonin levels from head extracts were determined by HPLC using an ESA model 582 isocratic pump, a Thermoseparation AS3500 autosampler (20 μl) and detection with an ESA CoulArray detector with potentials set at 25, 500, 650, and 800 mV. A prodigy C185 μm (250 × 4.6 mm) column (Phenomenex) and a Phenomenex Securityguard C18 precolumn were used with a mobile phase of MDTM/acetonitrile (85:15); (MDTM = 75 mM NaH2PO4, 1.7 mM 1-octanesulfonic acid, 100 μl/liter triethylamine, 25 mM EDTA, adjusted to pH 3 with phosphoric acid) pumped at 0.8 ml/min. Serotonin levels were quantified by using standard curves generated in parallel (serotonin >98% purity; Sigma). Octopamine levels were detected by using the same HPLC column, but with a mobile phase of 50 mM citrate acetate buffer, 11 mM octanesulfonic acid, pH 4.5/acetonitrile (80:20) (after ref. 56), and quantified by comparing peak areas to a standard curve (octopamine >95% purity; Sigma). Dopamine levels were determined from head extracts by using an enzyme immunoassay kit per the manufacturer's instructions (Labor Diagnostika Nord).
Microscopy.
Fly brains were extracted and blocked as described (57). A monoclonal antibody against serotonin (1:50; Biomeda) and/or anti-GFP (1:100; Sigma) was incubated with fixed brains overnight at 4°C. Secondary antibodies [Alexa 488 goat anti-rabbit (1:100; Invitrogen) and Alexa 647 goat anti-mouse (1:250; Invitrogen)] were incubated again with brains overnight at 4°C. All antibody incubation steps were followed by three 10-min washes with a modified PBS (57). Brains were mounted on slides by using vectashield and imaged with a Zeiss LSM Meta N2O two-photon inverted microscope. The whole brain was scanned and image stacks were analyzed with LSM (Zeiss) and Photoshop (Adobe) software. Depth coding used the DepthCod option from the LSM software. Image stacks were sometimes grouped according to anterior/posterior parts of the fly brain (anterior is used here to describe brain regions at the level of the fan-shaped body and rostral).
Behavioral Tests.
Flies were trained in the heat box as described (17, 19). Briefly, during training, when an individual fly crossed an invisible midline, the chamber heated up, reaching a maximal temperature of 41°C within seconds. When that fly recrossed the midline, the chamber cooled and approached 24°C. Training sessions of 20 min were used. After training, a memory test of 3 min measured the persistent avoidance of the chamber position associated with the high temperature. Avoidance behavior was quantified by using a performance index (16, 58), in which the time spent on the punishment-associated chamber half was subtracted from the time in the unpunished half, all divided by the total time. The performance index ranges from −1 to 1, with 1 being perfect avoidance of the chamber-half associated with high temperature. A performance index value of 0 indicates no side preference. The front and rear half of the chambers are associated with high temperature in an equal number of experiments. As performance index scores from place memory (and thermosensitivity) experiments give mixed results from tests on normality (15), nonparametric statistics are used for comparing memory levels between genotypes.
We refer to the test for the ability of flies to sense and avoid a high-temperature source as the thermosensitivity assay (18, 59). These tests use the same chambers; the difference is that the temperature of each chamber half is manipulated independently of a given fly's behavior. After 1 min when both chamber halves are held at 24°C, one chamber half is warmed to 41°C. A performance index is calculated in the same fashion as in the learning experiment. Again, an equal number of experiments started with the 41°C side in the front or back of the chamber. A second control we examined is the relationship between walking activity and memory performance. We have yet to detect a significant relationship in different genotypes between walking activity and the ability to form memory (D.S. and T.Z., unpublished results) (19).
Acknowledgments.
We thank the Bloomington Drosophila Stock Center (Bloomington, IN), James Birchler (University of Missouri), Serge Birman (Centre National de la Recherche Scientifique, Marseille, France), Henrike Scholz (University of Wuerzburg, Wuerzburg, Germany), and Dean Smith (University of Texas Southwestern, Dallas) for fly lines; Jonathan W. King, Andrew McClellan, and David Schulz for careful reading of an earlier version of the manuscript; and members of the University of Missouri Molecular Cytology Core for help with microscopy. This work was supported by the University of Missouri Research Council and Research Board and National Science Foundation Grant IOB 0613708 (to T.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710168105/DCSupplemental.
References
- 1.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 2.Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science. 2004;304:1782–1787. doi: 10.1126/science.1094765. [DOI] [PubMed] [Google Scholar]
- 3.Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
- 5.Baxter DA, Byrne JH. Feeding behavior of Aplysia: A model system for comparing cellular mechanisms of classical and operant conditioning. Learn Mem. 2006;13:669–680. doi: 10.1101/lm.339206. [DOI] [PubMed] [Google Scholar]
- 6.Unoki S, Matsumoto Y, Mizunami M. Roles of octopaminergic and dopaminergic neurons in mediating reward and punishment signals in insect visual learning. Eur J Neurosci. 2006;24:2031–2038. doi: 10.1111/j.1460-9568.2006.05099.x. [DOI] [PubMed] [Google Scholar]
- 7.Schwaerzel M, et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unoki S, Matsumoto Y, Mizunami M. Participation of octopaminergic reward system and dopaminergic punishment system in insect olfactory learning revealed by pharmacological study. Eur J Neurosci. 2005;22:1409–1416. doi: 10.1111/j.1460-9568.2005.04318.x. [DOI] [PubMed] [Google Scholar]
- 9.Schroll C, et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Menzel R, Heyne A, Kinzel C, Gerber B, Fiala A. Pharmacological dissociation between the reinforcing, sensitizing, and response-releasing functions of reward in honeybee classical conditioning. Behav Neurosci. 1999;113:744–754. [PubMed] [Google Scholar]
- 11.Tempel BL, Livingstone MS, Quinn WG. Mutations in the dopa decarboxylase gene affect learning in Drosophila. Proc Natl Acad Sci USA. 1984;81:3577–3581. doi: 10.1073/pnas.81.11.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsh J. Molecular genetics of dopa decarboxylase and biogenic amines in Drosophila. Dev Genet. 1989;10:232–238. doi: 10.1002/dvg.1020100312. [DOI] [PubMed] [Google Scholar]
- 13.Blenau W, Baumann A. Molecular and pharmacological properties of insect biogenic amine receptors: Lessons from Drosophila melanogaster and Apis mellifera. Arch Insect Biochem Physiol. 2001;48:13–38. doi: 10.1002/arch.1055. [DOI] [PubMed] [Google Scholar]
- 14.Wustmann G, Heisenberg M. Behavioral manipulation of retrieval in a spatial memory task for Drosophila melanogaster. Learn Mem. 1997;4:328–336. doi: 10.1101/lm.4.4.328. [DOI] [PubMed] [Google Scholar]
- 15.Putz G, Heisenberg M. Memories in Drosophila heat-box learning. Learn Mem. 2002;9:349–359. doi: 10.1101/lm.50402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wustmann G, Rein K, Wolf R, Heisenberg M. A new paradigm for operant conditioning of Drosophila melanogaster. J Comp Physiol A. 1996;179:429–436. doi: 10.1007/BF00194996. [DOI] [PubMed] [Google Scholar]
- 17.Zars T, Wolf R, Davis R, Heisenberg M. Tissue-specific expression of a type I adenylyl cyclase rescues the rutabaga mutant memory defect: In search of the engram. Learn Mem. 2000;7:18–31. doi: 10.1101/lm.7.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zars M, Zars T. High and low temperatures have unequal reinforcing properties in Drosophila spatial learning. J Comp Physiol A. 2006;192:727–735. doi: 10.1007/s00359-006-0109-6. [DOI] [PubMed] [Google Scholar]
- 19.Diegelmann S, Zars M, Zars T. Genetic dissociation of acquisition and memory strength in the heat-box spatial learning paradigm in Drosophila. Learn Mem. 2006;13:72–83. doi: 10.1101/lm.45506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan DT, Bell LA, Paton DR, Sullivan MC. Genetic and functional analysis of tryptophan transport in Malpighian tubules of Drosophila. Biochem Genet. 1980;18:1109–1130. doi: 10.1007/BF00484342. [DOI] [PubMed] [Google Scholar]
- 21.Tearle RG, Belote JM, McKeown M, Baker BS, Howells AJ. Cloning and characterization of the scarlet gene of Drosophila melanogaster. Genetics. 1989;122:595–606. doi: 10.1093/genetics/122.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dreesen TD, Johnson DH, Henikoff S. The brown protein of Drosophila melanogaster is similar to the white protein and to components of active transport complexes. Mol Cell Biol. 1988;8:5206–5215. doi: 10.1128/mcb.8.12.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funderburk CD, Bowling KM, Xu D, Huang Z, O'Donnell JM. Atypical N-terminal extensions confer novel regulatory properties on GTP cyclohydrolase isoforms in Drosophila melanogaster. J Biol Chem. 2006;281:33302–33312. doi: 10.1074/jbc.M602196200. [DOI] [PubMed] [Google Scholar]
- 24.Fitzpatrick PF. Mechanism of aromatic amino acid hydroxylation. Biochemistry. 2003;42:14083–14091. doi: 10.1021/bi035656u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnakumar S, Burton D, Rasco J, Chen X, O'Donnell J. Functional interactions between GTP cyclohydrolase I and tyrosine hydroxylase in Drosophila. J Neurogenet. 2000;14:1–23. doi: 10.3109/01677060009083474. [DOI] [PubMed] [Google Scholar]
- 26.Hammer M, Menzel R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn Mem. 1998;5:146–156. [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Chaney S, Roberts IJ, Forte M, Hirsh J. Ectopic G proteinexpression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr Biol. 2000;10:211–214. doi: 10.1016/s0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- 28.Friggi-Grelin F, et al. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 29.Kalidas S, Smith DP. Novel genomic cDNA hybrids produce effective RNA interference in adult Drosophila. Neuron. 2002;33:177–184. doi: 10.1016/s0896-6273(02)00560-3. [DOI] [PubMed] [Google Scholar]
- 30.Campbell JL, Nash HA. Volatile general anesthetics reveal a neurobiological role for the white and brown genes of Drosophila melanogaster. J Neurobiol. 2001;49:339–349. doi: 10.1002/neu.10009. [DOI] [PubMed] [Google Scholar]
- 31.Pal-Bhadra M, Bhadra U, Birchler JA. Cosuppression in Drosophila: Gene silencing of Alcohol dehydrogenase by white-Adh transgenes is Polycomb dependent. Cell. 1997;90:479–490. doi: 10.1016/s0092-8674(00)80508-5. [DOI] [PubMed] [Google Scholar]
- 32.Martin JR, Keller A, Sweeney ST. Targeted expression of tetanus toxin: A new tool to study the neurobiology of behavior. Adv Genet. 2002;47:1–47. doi: 10.1016/s0065-2660(02)47001-0. [DOI] [PubMed] [Google Scholar]
- 33.Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 34.Lee T, Luo L. Mosaic analysis with a repressible neurotechnique cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 35.Valles AM, White K. Serotonin-containing neurons in Drosophila melanogaster: Development and distribution. J Comp Neurol. 1988;268:414–428. doi: 10.1002/cne.902680310. [DOI] [PubMed] [Google Scholar]
- 36.Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet. 2007;39:678–682. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
- 37.Marican C, Duportets L, Birman S, Jallon JM. Female-specific regulation of cuticular hydrocarbon biosynthesis by dopamine in Drosophila melanogaster. Insect Biochem Mol Biol. 2004;34:823–830. doi: 10.1016/j.ibmb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Herrnstein RJ. The Matching Law: Papers in Psychology and Economics. Cambridge, MA: Harvard Univ Press; 1997. [Google Scholar]
- 39.Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioral control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- 40.Klein TA, et al. Genetically determined differences in learning from errors. Science. 2007;318:1642–1645. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- 41.Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Holmes A, Lachowicz JE, Sibley DR. Phenotypic analysis of dopamine receptor knockout mice: Recent insights into the functional specificity of dopamine receptor subtypes. Neuropharmacology. 2004;47:1117–1134. doi: 10.1016/j.neuropharm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 44.Sarnyai Z, et al. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc Natl Acad Sci USA. 2000;97:14731–14736. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malleret G, Hen R, Guillou JL, Segu L, Buhot MC. 5-HT1B receptor knockoutmice exhibit increased exploratory activity and enhanced spatial memory performance in the Morris water maze. J Neurosci. 1999;19:6157–6168. doi: 10.1523/JNEUROSCI.19-14-06157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hing AL, Carlson JR. Male-male courtship behavior induced by ectopic expression of the Drosophila white gene: Role of sensory function and age. J Neurobiol. 1996;30:454–464. doi: 10.1002/(SICI)1097-4695(199608)30:4<454::AID-NEU2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 47.Zhang SD, Odenwald WF. Misexpression of the white (w) gene triggers male-male courtship in Drosophila. Proc Natl Acad Sci USA. 1995;92:5525–5529. doi: 10.1073/pnas.92.12.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoyer SC, et al. Octopamine in male aggression of Drosophila. Curr Biol. 2008;18:159–167. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem. 2000;7:170–179. doi: 10.1101/lm.7.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 51.Lein ES, et al. Genomewide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 52.Tachikawa M, et al. Distinct spatio-temporal expression of ABCA and ABCG transporters in the developing and adult mouse brain. J Neurochem. 2005;95:294–304. doi: 10.1111/j.1471-4159.2005.03369.x. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura M, Ueno S, Sano A, Tanabe H. Polymorphisms of the human homologue of the Drosophila white gene are associated with mood and panic disorders. Mol Psychiatry. 1999;4:155–162. doi: 10.1038/sj.mp.4000515. [DOI] [PubMed] [Google Scholar]
- 54.Wolf R, Heisenberg M. Basic organization of operant behavior as revealed in Drosophila flight orientation. J Comp Physiol A. 1991;169:699–705. doi: 10.1007/BF00194898. [DOI] [PubMed] [Google Scholar]
- 55.Scholz H, Ramond J, Singh CM, Heberlein U. Functional ethanol tolerance in Drosophila. Neuron. 2000;28:261–271. doi: 10.1016/s0896-6273(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 56.Hardie SL, Hirsh J. An improved method for the separation and detection of biogenic amines in adult Drosophila brain extracts by high performance liquid chromatography. J Neurosci Methods. 2006;153:243–249. doi: 10.1016/j.jneumeth.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Rein K, Zockler M, Mader MT, Grubel C, Heisenberg M. The Drosophila standard brain. Curr Biol. 2002;12:227–231. doi: 10.1016/s0960-9822(02)00656-5. [DOI] [PubMed] [Google Scholar]
- 58.Dill M, Wolf R, Heisenberg M. Visual pattern recognition in Drosophila involves retinotopic matching. Nature. 1993;365:751–753. doi: 10.1038/365751a0. [DOI] [PubMed] [Google Scholar]
- 59.Zars T. Two thermosensors in Drosophila have different behavioral functions. J Comp Physiol A. 2001;187:235–242. doi: 10.1007/s003590100194. [DOI] [PubMed] [Google Scholar]