Abstract
Pre-fight displays typically provide honest, but sometimes dishonest, information about resource holding potential and may be influenced by assessment of resource value and hence motivation to acquire the resource. These assessments of potential costs and benefits are also predicted to influence escalated fight behaviour. This is examined in shell exchange contests of hermit crabs in which we establish an information asymmetry about a particularly poor quality shell. The poor shell was created by gluing sand to the interior whereas control shells lacked sand and the low value of the poor shell could not be accurately assessed by the opponent. Crabs in the poor shell showed changes in the use of pre-fight displays, apparently to increase the chances of swapping shells. When the fights escalated, crabs in poor shells fought harder if they took the role of attacker but gave up quickly if in the defender role. These tactics appear to be adaptive but do not result in a major shift in the roles taken or outcome. We thus link resource assessment with pre-fight displays, the roles taken, tactics used during escalation and the outcome of these contests.
Keywords: assessment, displays, hermit crab, fighting behaviour, resource value
1. Introduction
Animal contests frequently start with displays and subsequently may proceed to escalated fighting. Displays may provide a mechanism for contest resolution without the need for costly physical combat by providing information about resource holding potential (RHP) or intent (Parker 1974; Maynard-Smith & Harper 2003; Searcy & Nowicki 2005). There has been a debate over the level of reliability to be expected. Some authors suggest that individuals attempt to manipulate opponents (Krebs & Dawkins 1984), whereas others suggest honest displays predicted by handicap theory (Zahavi 1975; Johnstone 1998) or signals that are a non-fakable index of an animal's size or condition and thus RHP (Maynard-Smith & Harper 2003). Early game theory models (e.g. Maynard-Smith & Parker 1976; Maynard-Smith 1979) suggested that threat displays conveying accurate information about aggressiveness (intent) or RHP could not be evolutionarily stable. However, later models, incorporating handicap theory (Zahavi 1975), showed that honest signalling was probable (e.g. Enquist 1985; Grafen 1990), but more recent models show that honest and deceitful signals can coexist in a stable system (e.g. Adams & Mesterton-Gibbons 1995; Szamado 2000). This debate (Johnstone 1998; Hurd & Enquist 2005) and subsequent empirical studies have focused mainly on displays that appear to advertise RHP. There are examples in which a contestant benefits from exaggerating or ‘bluffing’ about RHP (Adams & Caldwell 1990; Backwell et al. 2000; Hughes 2000) or possibly from disrupting attempts to assess RHP (Elwood et al. 2006). Studies considering motivational signals of intent when animals have ‘private’ or ‘personal’ information (Dall et al. 2005) concerning the true value of a resource are fewer in number. Enquist et al. (1985) showed that in Northern Fulmars (Fulmarus glacialis) resource value-influenced signal choice so that a more effective and costly option was chosen when the value increased, thus acting as an honest display of the signaller intent (also see Hansen 1986).
Selection should also favour contestants that modify their behaviour during escalated fights following resource value assessment (Parker 1974; Maynard-Smith & Parker 1976; Parker & Stuart 1976). Resource assessment is inferred from a positive relationship between the resource value and the costs that contestants are prepared to pay (Parker 1974; Maynard-Smith & Parker 1976; Parker & Stuart 1976; Enquist & Leimar 1987). Costs are estimated by contest duration (e.g. Verrell 1986), vigour (e.g. Briffa et al. 1998) or physiological change such as increase in lactate or reduction of energy stores (e.g. Briffa & Elwood 2001, 2002, 2004; Prenter et al. 2006). Alternatively, resource assessment is inferred from changes in the probability of victory (e.g. Humphries et al. 2006).
The present study investigates resource assessment in the hermit crab, Pagurus bernhardus, contesting ownership of shells. Fights over shell occupancy are typically preceded by displays of chelipeds (claws) and walking legs and also a high posture in which the shell is lifted high off the substrate (Elwood et al. 2006). The larger crab is more prone to use ‘cheliped presentation’ as a display, which appears to convey accurate size information, whereas the smaller is more prone to use cheliped extension, which is less likely to provide accurate size information. Smaller crabs that use the extension display to a great extent are less likely to be attacked and less likely to be evicted, than those smaller crabs employing low levels of extension. Each contestant preferentially uses displays that maximize its success rather than being essentially honest (Elwood et al. 2006).
This pre-fight phase may be followed by an escalated fight in which one crab, termed the ‘attacker’, approaches and grasps the shell of the defender, causing the defender to withdraw into its shell (Dowds & Elwood 1983). The attacker may then engage in repeated bouts of vigorous shell rapping in which the attacker hits its shell upon that of the defender until either the defender is evicted from the shell, enabling the attacker to take that shell, or the attacker gives up. Thus, it is only in the attacker role that a crab has the chance of choosing between alternative shells and normally, the larger of the two crabs takes this advantageous role. In the species of the present study, it is the attacker that normally gathers accurate information about the quality of the opponent's shell by feeling over the external surface, but because the defender withdraws into its shell the defender cannot assess the attacker's shell (Elwood & Neil 1992). Here, however, we create a situation for a treatment group where the smaller crab is in a shell that has rough sand glued to the inside such that the smaller crab has information that it is a poor shell but that information is hidden from the larger crab. Crabs appear to find sand in the shell aversive as they will remove loose sand and attempt to scrape off fixed sand (Elwood & Adams 1990). A control group lacks the sand. The larger crab is expected to have no information about the sand and should initially value the opponent's shell equally in the two groups. The smaller crab, however, has private or personal information (Dall et al. 2005) and is expected to value shells with sand less than they will control shells and thus crabs in these ‘sandy’ shells may engage in tactics by which it can increase the probability of exchange in a shell fight. It can potentially get rid of this poor shell in one of two ways. First, it can take the role of attacker and evict its opponent, in which case it can choose between the two shells. Second, it may take the role of defender and allow itself to be evicted and thus may attempt to affect a shell exchange with the opponent if the ‘winner’ does not immediately note the sand and choose to go back to its original shell. In this species, the winner typically holds on to its old shell while ‘trying out’ the new shell and may move back and forth between the shells prior to making a decision (Elwood & Neil 1992). We determine whether sand: (i) influences pre-fight display behaviour and subsequent tactical decisions, (ii) results in the smaller crab being more likely to enter a fight (either as attacker or defender) rather than avoid a fight, (iii) influences the fight tactics of the smaller crab during the escalated phase, being more persistent if it takes the role of attacker, but giving up early if it takes the role of defender, and (iv) results in a shift in fight outcome.
2. Material and methods
(a) Collection and maintenance of specimens
Small (0.10–0.36 g) littoral specimens of the common European hermit crab P. bernhardus were collected weekly from the shore at Ballywalter, Co. Down, Northern Ireland, between June and December 2006. Specimens were kept in groups of up to 75 in 60 cm×30 cm plastic tanks, filled with aerated seawater to a depth of 10 cm, at 12°C with a 12 hours day/night regime, and fed ad libitum on commercial fish food ‘catfish pellets’. Crabs were removed from their shells by cracking the shells open using a small bench vice in such a way that no crabs were harmed. Each crab was then sexed (based on the number, position and morphology of abdominal pleopods, Elwood & Neil 1992), and males only were used in the study. Females were supplied with new shells, and returned to the sea, thus avoiding sex differences in behaviour that have been noted in previous studies (Neil & Elwood 1985). Only male crabs that were free from loss of appendages, obvious parasites and recent moult were used.
(b) Preference test
A shell preference test was carried out to ascertain whether crabs prefer shells with no sand inside them as opposed to shells with sand fixed to the inner whorls of the shell. Littorina obtusata shells were collected from the upper shore of Portaferry, Co. Down, Northern Ireland. Any holed or fragmented shells were discarded, while the remainder were washed with boiling water to remove debris and epibionts. A small volume of commercial water-resistant glue (‘Extreme repair’ from Unibond) was placed in the interior of the shell and a paintbrush used to work the glue deep into the interior. Sand was then poured into the shell aperture and the shell rotated in an anticlockwise direction about the columella axis to force sand deep into the shell interior. The shell was then left for 3 min, aperture upwards filled with sand, before being lifted and rotated in the opposite direction to free any loose sand. Other shells were treated with glue but no sand, to control for the presence of potentially influential chemicals in the glue and thus ensure that the only treatment difference was the presence or absence of sand. All the shells were then left for at least 48 hours to allow the glue to dry, and then reweighed. A fine marker pen was used to identify shells.
Six L. obtusata shells were used for each replicate of a preference test, three shells containing sand and three containing glue only. One of each type was the preferred weight for the crab as determined using previously calculated regression lines that relate crab weight to preferred shell weight (Jackson 1988), one of each of the three was 10% greater than the preferred weight and one of each of the three was 10% less than the preferred weight.
Twenty weighed male crabs were each matched to an appropriate set of six shells. The shells were then placed aperture upwards in a 12 cm diameter, 765 ml plastic dish containing 300 ml aerated seawater, and a small dissection pointer was used to displace any trapped air bubbles. The naked male crab was then placed with the six shells and the shell occupied after a 2 hours period was recorded. The data were examined using a chi-squared goodness-of-fit test.
(c) Contests
Crabs were collected and held as above and each male was weighed and placed ‘naked’ in an individual 12 cm diameter 765 ml plastic dish containing 300 ml aerated seawater. Crabs were then allocated to pairs so that the larger of the two was no more than 10% larger than its opponent. The relative weight difference (RWD) of the pair was calculated by RWD=1−(small crab weight/large crab weight). The pairs were then allocated randomly to one of the two treatment groups based on the toss of a coin. The preferred weight of shell for the larger crab of each pair was determined as per Jackson (1988) and in all cases the smaller crab of each pair was provided with a L. obtusata shell that was matched to the preferred weight for the larger crab. In the ‘control’ group, the smaller crab was given a shell that contained glue only and in the sandy group the smaller contestant of each pair was provided with a shell that had sand in the inner whorls. In both the groups, the larger crab of each pair was given a L. obtusata shell that was 50% of its preferred weight (n=60 for each group).
Each crab was isolated with its new shell in a 12 cm diameter 765 ml plastic dish containing 300 ml aerated seawater at 12 °C for 3 hours prior to the contest being staged. The interactions were observed in a 14 cm diameter glass bowl containing 350 ml aerated seawater and a 3 cm deep layer of aquarium gravel. The crabs were separated in the contest arena by placing them inside two clear plastic cylinders (3.7 cm diameter and 5.5 cm height) positioned such that the cylinders were touching, enabling visual contact for 2 min before the cylinders were removed and the observation started. A camcorder was used to record the subsequent interactions and then analysed using a Psion Workabout hand-held computer configured as a time-event recorder using the Observer v. 3.0 software (Noldus Technology, Wageningen, The Netherlands). Interactions were allowed to continue until the attacker either evicted the defender or gave up without effecting an eviction or, if a fight was not initiated, after 30 min.
We recorded pre-fight displays including: ‘cheliped presentation’ (the proximal part of the chelipeds is held forward, towards the opponent, with the distal part (claw) being approximately perpendicular to the substrate); ‘cheliped extension’ (the major, often with the minor, cheliped is moved forwards with the claw(s) approximately horizontal to the substrate and raised up at least to the level of the head of the displayer, typically with the chela(e) open); ‘ambulatory raise’ (at least one of the walking legs is raised and extended sideways away from the body and held above the substrate; Elwood & Neil 1992); ‘grapple’ (mutual wrestling with the chelipeds and walking legs); ‘high posture’ (both the body of the crab and shell raised off the substrate); ‘approach’ (movement towards the opponent); and ‘retreat’ (movement away from the opponent). If a shell fight occurred, the pattern of shell rapping, duration and outcome was also recorded. After the interaction, the crabs were provided with suitable shells and returned to the collection site.
(d) Statistical methods
Categorical data were investigated using Χ2-tests. A series of t-tests and two-way ANOVAs were used to examine any behavioural differences between the treatment groups. Principal components analysis (PCA) was used to reduce the number of pre-fight display activities into a more manageable set of components. We consider only those activities with loadings of greater than 0.6 in each component (Frey & Pimental 1978). The component scores were then analysed by two-way ANOVA. Fights differing in outcome (either eviction or non-eviction) were analysed separately using two-way ANOVA (treatment group and attacker) for cases that led to eviction. For those fights not leading to eviction there were too few fights in which the larger attacker failed to evict the defender to conduct a two-way ANOVA, therefore a t-test was used. Data were log(n+1)-transformed as appropriate. There was no difference between the two treatment groups for small crab weight (control: mean±s.e.=0.210 g±0.007; sandy: mean±s.e.=0.208 g±0.008, t118=0.133, p=0.895) or large crab weight (control: mean±s.e.=0.225 g±0.008; sandy: mean±s.e.=0.225 g±0.009, t118=0.67, p=0.947). Shells with sand weighed 6% more than did those with glue only (control: mean±s.e.=1.231 g±0.019; sandy: mean±s.e.=1.305 g±0.022, t118=−2.550, p=0.012).
3. Results
In the first experiment there was a strong preference for control shells rather than shells with sand glued to the inside of them (18 versus 2; goodness-of-fit Χ12=12.8, p<0.0001).
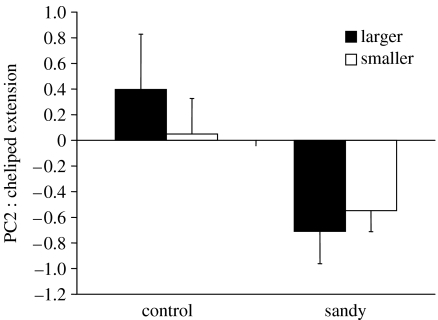
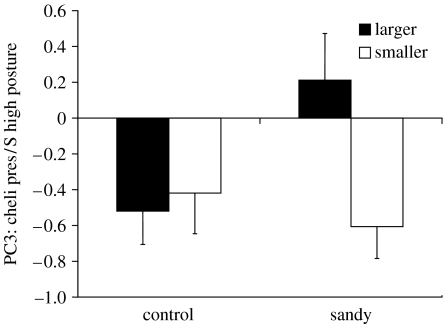
In the second experiment, pre-fight duration did not differ between the treatment groups (F1,57=0.701, p=0.406) and there was no effect of which crab (larger or smaller) was the attacker (F1,57=0.444, p=0.508) nor was there a significant interaction effect (F1,57=0.199, p=0.658). PCA of the nine pre-fight displays (excluding approach and retreat) yielded four components (table 1). PC1 comprised ambulatory raise and high posture by the large crab; PC2 comprised cheliped extension by both the crabs; PC3 comprised cheliped presentation by both crabs and high posture by the small crab; and PC4 comprised grappling. For PC1 component scores there was no group effect (F1,57=0.878, p=0.353), no effect as to which crab was the attacker (F1,57=2.460, p=0.122) and no interaction (F1,57=0.017, p=0.898). For PC2, however, there was a significant group effect (F1,57=9.425, p=0.0033; figure 1) with crabs from the sandy treatment having lower component scores, i.e. both the crabs showed lower amounts of cheliped extension. There was no effect as to which crab was the attacker (F1,57=0.112, p=0.739), and no interaction effect (F1,57=0.840, p=0.363). For PC3 there was no group effect (F1,57=1.415, p=0.239), and no effect of which crab was the attacker (F1,57=2.446, p=0.1234). However, there was a significant interaction effect (F1,57=4.021, p=0.0497; figure 2) with fights in which the larger crab took the role of attacker in the sandy group using more cheliped presentation than of those in the other groups, particularly of those when the smaller crab took the attacker role in the sandy group. For PC4 there was no group effect (F1,57=0.271, p=0.605), no effect as to which crab was the attacker (F1,57=0.513, p=0.477) and no interaction effect (F1,57=0.004, p=0.947).
Table 1.
Loadings of pre-fight displays on to the four principal components. (Only those activities with loadings of greater than 0.6 in each component were considered (shown in italic; S, smaller crab; L, larger crab).)
| pre-fight display | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| S cheliped extension | 0.146 | 0.749 | −0.011 | −0.001 |
| S cheliped presentation | 0.051 | 0.242 | 0.600 | 0.001 |
| S high posture | 0.012 | −0.206 | 0.674 | 0.198 |
| grapple | −0.055 | 0.218 | 0.009 | 0.738 |
| S ambulatory raise | 0.426 | 0.000 | 0.406 | −0.236 |
| L cheliped extension | −0.114 | 0.759 | 0.038 | 0.133 |
| L cheliped presentation | −0.001 | 0.216 | 0.697 | −0.120 |
| L high posture | 0.757 | −0.189 | −0.040 | 0.435 |
| L ambulatory raise | 0.630 | 0.138 | 0.001 | −0.161 |
Figure 1.
Mean (±s.e.) component scores for PC2 (cheliped extension) for the ‘control’ and ‘sandy’ treatments in which either the larger or the smaller animal initiated the fight.
Figure 2.
Mean (±s.e.) component scoress for PC3 (cheliped presentation and small crab high posture) for the ‘control’ and ‘sandy’ treatments in which either the larger or the smaller animal initiated the fight.
There was no significant difference between the two treatment groups in the occurrence of escalated fights (Χ12=0.133, p=0.715; table 2). Of those fights, there was no difference between the treatment groups in the probability of the larger crab taking the role of attacker (Χ12=0.232, p=0.630; table 2) and there was no difference between the treatments as to whether an eviction occurred or not (Χ12=0.120, p=0.729; table 1). There was no difference in the probability that the larger or the smaller crab took the role of attacker (Χ12=2.77, p<0.1; table 2).
Table 2.
Summary of data for fights.
| treatment | no. of fights | initiation by larger animal | initiation by smaller animal | evictions by larger animal | non-evictions by larger animal | evictions by smaller animal | non-evictions by smaller animal |
|---|---|---|---|---|---|---|---|
| control (n=60) | 29 | 10 | 19 | 8 | 2 | 7 | 12 |
| sandy (n=60) | 32 | 14 | 18 | 13 | 1 | 6 | 12 |
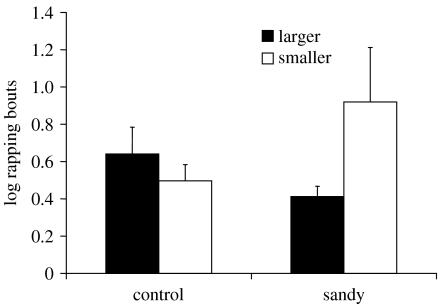
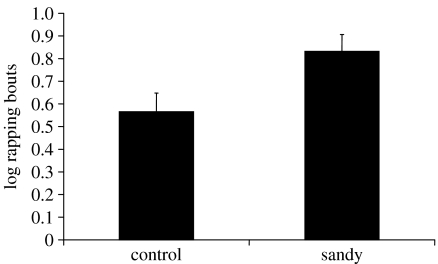
For fights that led to an eviction, the number of bouts of rapping revealed no effect of group (F1,30=0.486, p=0.491) and there was no effect as to which crab was the attacker (F1,30=1.720, p=0.200) but, there was a significant interaction effect (F1,30=5.499, p=0.026; figure 3) with larger attackers using few bouts of rapping to evict smaller defenders in the sandy group, and smaller attackers taking many bouts of rapping to evict larger defenders in the sandy group. In cases of non-eviction, there were too few fights in which the larger attacker failed to evict the defender to conduct a two-way ANOVA (table 2). However, for smaller attackers that failed to evict, there were significantly more bouts of rapping (log(n+1)) by those in the sandy group than in the control group (sandy: mean±s.e.=0.832±0.074; control: mean±s.e.=0.566±0.082, t22=−2.412, p=0.025).
Figure 3.
Mean (+s.e.) number of rapping bouts (log(n+1)) for the ‘control’ and ‘sandy’ treatments in which either the larger or the smaller animal initiated the fight, in cases which led to an eviction.
Larger attackers were more likely to evict the opponent than were smaller attackers (larger: 21/24; smaller: 13/37, Χ12=14.2, p=0.0002; table 2). There was no significant difference between the treatment groups in the ability of larger attackers to evict smaller defenders (larger attacker causing eviction: control 8/10; sandy: 13/14, Χ12=0.098, p=0.754) or of smaller attackers to evict larger defenders (smaller attacker causing eviction: control: 7/19; sandy: 6/18, Χ12=0.00, p=0.99) or increase the proportion of all fights ending in eviction (control: 15/29; sandy: 19/32, Χ12=0.361, p=0.584). RWD did not differ between treatment groups (F1,57=1.577, p=0.214) or between cases when the attacker was the larger or the smaller crab (F1,57=0.104, p=0.748) and there was no interaction effect (F1,57=0.004, p=0.949).
4. Discussion
The preference for shells without sand was clear. This might be due to the increased weight of sandy shells because heavy shells are less preferred (Briffa & Elwood 2005). However, the additional weight of the sand was slight and well within the natural variation in the weight of shells. We thus presume that the avoidance of sandy shells is primarily due to the rough interior, which may damage the delicate abdomen of these hermit crabs and thus they presumably had a high motivation to obtain a new shell. The sand was placed deep within the shell such that it could not be accessed by an opponent during a contest, and the negative aspect of the shell was private information to the crab inhabiting that shell. Furthermore, even if the additional weight was a factor in the avoidance of sandy shells this information would not be available to the opponent until a late stage of the contest.
Hermit crab fights are preceded by a period of display that influences which crab takes the role of attacker, this role typically going to the larger opponent (Elwood & Glass 1981; Dowds & Elwood 1983; Elwood & Neil 1992) but which crab takes that role is influenced by the pre-fight displays (Elwood et al. 2006). When the larger crab takes the attacker role there is a more cheliped presentation, which allows a clear assessment of relative cheliped size. When the smaller crab takes the attacker role it shows more cheliped extension, which may act to disrupt the assessment of cheliped size or may act to keep the larger crab at a distance and hence reduce the chance of a fight developing (Elwood et al. 2006). In the present study, the cheliped extension display was used much less in the sandy group and we presume this is an altered tactic of the smaller crabs because only it can know about the sand. Reducing the use of this display might indicate a reduced motivation to defend their shell. Enquist (1985) and Enquist et al. (1985) demonstrate that honest signals of intent (motivation) can occur but must contain costs to maintain reliability. In hermit crabs, however, the cost of these displays is not likely to be high (Elwood et al. 2006). Coupled with this reduced motivation to defend might be an increased willingness to take the role of defender in the ‘hope’ of an exchange of shells. However, despite the altered tactic, it does not significantly alter the role that is taken because there were low levels of extension display in the sandy group irrespective of which crab took the role of attacker.
The use of the cheliped presentation display did not differ between treatment groups but the significant interaction effect indicates that it is associated with the assumption of different roles in the sandy group. Within this group, when the larger crab took the role of attacker, there was more mutual presentation than when the smaller crab took that role. The presentation display allows mutual assessment of cheliped size (Elwood et al. 2006) and thus allows the larger crab to assess that it is the larger of the two contestants and thus take the role of attacker. In the sandy group, the marked difference in the use of the presentation display suggests that the smaller crab takes the attacker role only if this ‘honest’ presentation display is reduced. If the smaller crab wins the contest as an attacker it is able to choose between the two shells and thus the role of attacker should be advantageous, but only if they can evict their opponent and small crabs have a disadvantage in this respect. If they take the role of defender, however, they will determine whether and when they are evicted but that does not guarantee an exchange of shells because the winner may detect the sand and return to the original shell (Elwood & Neil 1992). In fact, while detailed analysis of shell occupancy following a fight was not conducted, in the vast majority of cases larger winners returned to their original shell after brief investigation of the sandy shell. Thus, only victory in the attacker role will definitely rid the smaller crab of a poor quality shell. The two components relating to non-cheliped displays did not differ between treatment groups or roles and thus the significant effects in the experiment are restricted to extension and presentation displays that use the chelipeds. Although a series of tests were applied, the close agreement with a previous study concerning the use of these two displays (Elwood et al. 2006) gives confidence that these are real effects.
Having shown changes in specific displays by the animal having private information about the true worth of this shell we also anticipate that this information will alter the tactics of those same animals during the subsequent escalated fight. Animals that place a higher value on a resource are expected to accept higher costs of escalated fighting (Parker 1974; Maynard-Smith & Parker 1976; Parker & Stuart 1976; Enquist & Leimar 1987), and the cost of a fight is frequently estimated by the time or number of specific activities taken to determine the winner (Taylor & Elwood 2003). Here we used the number of bouts of shell rapping as a proxy of cost because shell rapping has been shown to be costly (Briffa & Elwood 2001, 2002). However, the cost tells us about the motivation of the loser as that is the animal that reveals the cost it is willing to pay by giving up. The winner only partially reveals the cost it is prepared to pay as we only know that it is at least as much as that of the loser. Thus, we examined separately fights that ended in eviction or not. Small attackers in sandy shells that evicted their larger opponent had to fight hard to win (figure 3) and if they did not evict their opponent they persisted for much longer prior to giving up than did crabs in control shells (figure 4). This demonstrates that small attackers in sandy shells were more highly motivated to win the encounter and to obtain new shells than those in control shells. If they took the role of the defender, however, they gave up quickly, indicating that they were not prepared to pay high costs in resisting eviction from these poor quality shells (figure 3). This shows that private information available to the smaller crabs influenced fight tactics in a way that should maximize their chances of obtaining alternative shells or at least minimize costs when in the defender role.
Figure 4.
Mean (+s.e.) number of rapping bouts (log(n+1)) for fights in which the smaller crab was the attacker but failed to evict its larger opponent.
Several studies show that information asymmetries between opponents regarding resource value affect contest behaviour. Many have involved owner–intruder situations in which the owner is thought to be well informed about the value of the resource whereas the intruder lacks the opportunity to gather such information. For example, Bridge et al. (2000) found that in male orb-web spiders (Metellina mengei), the resident but not the intruder was able to adjust fight tactics with regard to female value. They found that in cases where the owner lost (intruder won) there was a positive correlation between female weight and both duration and intensity. By contrast, when the owner won (intruder lost), there was no effect of female weight, indicating that the intruder was not capable of assessing female value. Others have reported similar findings with regard to owner–intruder scenarios and assessment for a variety of resources (e.g. nesting burrows, Rand & Rand 1976; territories, Riechert 1979, 1984; females, Sigurjonsdottir & Parker 1981, Hack et al. 1997; cases, Englund & Otto 1991). Few studies have attempted to study information asymmetries by manipulating the resource to change its value in different ways for both the contestants (but see Humphries et al. 2006; Goubault et al. 2007). Many more studies have focused on varying subjective resource value between contestants by manipulating the internal state of one animal by depriving it of food and then allowing a contest for food. In those cases, hungry animals fight for longer (e.g. Popp 1987) and may significantly shift the probability of winning (e.g. Hansen 1986; Popp 1987; Crowley et al. 1988; Cristol 1992; Rodriguez-Girones et al. 1996; Nosil 2002). The effects of external resource value manipulations have also been inferred from changes in contest outcome (e.g. Lindstrom 1992; Kotiaho et al. 1999; Humphries et al. 2006). These studies, however, have not examined both pre-fight displays and escalated fight tactics.
In the present study, despite the shift in fight effort (bouts of rapping), there was no significant increase in shell exchanges in the sandy group. Thus, the change in fight tactics shown by smaller crabs in the sandy group was not sufficient to alter the outcome in their favour. Similarly, in contests between pumpkinseed sunfish (Lepomis gibbosus), Dugatkin & Ohlsen (1990) manipulated the expectation of food resource a given fish would receive and those with higher expectations attacked first significantly more often than those with lower expectations. However, there was no difference in the outcome, i.e. winning the contest was split equally between fish with higher and lower expectations. Hence studies that focus on changes in outcome without considering changes in fight cost or behaviour (e.g. contest duration or intensity) may fail to find an effect of resource value manipulation where one actually exists. In other words, manipulating resource value may alter the behaviour of contestants in a fight but with only a small, and hence typically non-significant, effect on outcome.
In conclusion, information asymmetries about resource value influenced the pre-fight extension display behaviour in a way consistent with crabs in poor shells being more willing to engage in a shell exchange contest. However, those crabs could only attain the attacker role if they reduced the amount of honest presentation displays, i.e. reduced the information about their true RHP. In escalated contests, smaller crabs in poor shells were more persistent when taking the role of attacker, but gave up early when taking the role of defender. We thus have clear support for predictions (i) and (iii) concerning contest behaviour due to altered motivation of one participant. However, contrary to predictions (ii) and (iv), the changes in fight behaviour did not result in the smaller crab being more likely to enter a fight and resulted only in a small, non-significant shift in outcome. Thus, although animals in contests may show significant shifts in tactics this may result in minor non-significant changes in success.
Acknowledgments
We thank the Department of Agriculture and Rural Development for funding and Gillian Riddell for field and laboratory assistance. Additional thanks go to Mark Laidre, Neil Metcalfe and three anonymous referees for their valuable comments and contributions to this manuscript.
References
- Adams E.S, Caldwell R.L. Deceptive communication in asymmetric fights of the stomatopod crustacean Gonodactylus bredini. Anim. Behav. 1990;39:706–716. doi:10.1016/S0003-3472(05)80382-3 [Google Scholar]
- Adams E.S, Mesterton-Gibbons M. The cost of threat displays and the stability of deceptive communication. J. Theor. Biol. 1995;175:405–421. doi:10.1006/jtbi.1995.0151 [Google Scholar]
- Backwell P.R.Y, Christy J.H, Telford S.R, Jennions M.D, Passmore N.I. Dishonest signalling in a fiddler crab. Proc. R. Soc. B. 2000;267:719–724. doi: 10.1098/rspb.2000.1062. doi:10.1098/rspb.2000.1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge A.P, Elwood R.W, Dick J.T.A. Imperfect assessment and limited information preclude optimal strategies in male–male fights in the orb-weaving spider Metellina mengei. Proc. R. Soc. B. 2000;267:273–279. doi: 10.1098/rspb.2000.0997. doi:10.1098/rspb.2000.0997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briffa M, Elwood R.W. Decision rules, energy metabolism and vigour of hermit-crab fights. Proc. R. Soc. B. 2001;268:1841–1848. doi: 10.1098/rspb.2001.1752. doi:10.1098/rspb.2001.1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briffa M, Elwood R.W. Power of shell-rapping signals influences physiological costs and subsequent decisions during hermit crab fights. Proc. R. Soc. B. 2002;269:2331–2336. doi: 10.1098/rspb.2002.2158. doi:10.1098/rspb.2002.2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briffa M, Elwood R.W. Use of energy reserves in fighting hermit crabs. Proc. R. Soc. B. 2004;271:373–379. doi: 10.1098/rspb.2003.2633. doi:10.1098/rspb.2003.2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briffa M, Elwood R.W. Metabolic consequences of shell choice in Pagurus bernhardus: do hermit crabs prefer cryptic or portable shells? Behav. Ecol. Sociobiol. 2005;59:143–148. doi:10.1007/s00265-005-0020-0 [Google Scholar]
- Briffa M, Elwood R.W, Dick J.T.A. Analysis of repeated signals during shell fights in the hermit crab Pagurus bernhardus. Proc. R. Soc. B. 1998;265:1467–1474. doi:10.1098/rspb.1998.0459 [Google Scholar]
- Cristol D.A. Food-deprivation influences dominance status in dark-eyed juncos, Junco hyemalis. Anim. Behav. 1992;43:117–124. doi:10.1016/S0003-3472(05)80077-6 [Google Scholar]
- Crowley P.H, Gillett S, Lawton J.H. Contests between larval damselflies: empirical steps toward a better ESS model. Anim. Behav. 1988;36:1496–1510. doi:10.1016/S0003-3472(88)80220-3 [Google Scholar]
- Dall S.R.X, Giraldeau L, Olsson O, McNamara J.M, Stephens D.W. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 2005;20:187–193. doi: 10.1016/j.tree.2005.01.010. doi:10.1016/j.tree.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Dowds B.M, Elwood R.W. Shell wars: assessment strategies and the timing of decisions in hermit crab shell fights. Behaviour. 1983;85:1–24. [Google Scholar]
- Dugatkin L.A, Ohlsen S.R. Contrasting asymmetries in value expectation and resource holding power: effects on attack behavior and dominance in the pumpkinseed sunfish, Lepomis gibbosus. Anim. Behav. 1990;39:802–804. doi:10.1016/S0003-3472(05)80394-X [Google Scholar]
- Elwood R.W, Adams P.M. How hermit crabs Pagurus bernhardus L. deal with obstructions in the aperture of shells. Ir. Nat. J. 1990;23:180–185. [Google Scholar]
- Elwood R.W, Glass C.W. Negotiation or aggression during shell fights of the hermit crab Pagurus bernhardus. Anim. Behav. 1981;29:1239–1244. doi:10.1016/S0003-3472(81)80075-9 [Google Scholar]
- Elwood R.W, Neil S.J. 1st edn. Chapman and Hall; London, UK: 1992. Assessment and decisions: a study of information gathering by hermit crabs. [Google Scholar]
- Elwood R.W, Pothanikat R.M.E, Briffa M. Honest and dishonest displays, motivational state and subsequent decisions in hermit crab shell fights. Anim. Behav. 2006;72:853–859. doi:10.1016/j.anbehav.2006.01.025 [Google Scholar]
- Englund G, Otto C. Effects of ownership status, weight asymmetry, and case fit on the outcome of case contests in 2 populations of Agrypnia pagetana (Trichoptera, Phryganeidae) larvae. Behav. Ecol. Sociobiol. 1991;29:113–120. doi:10.1007/BF00166485 [Google Scholar]
- Enquist M. Communication during aggressive interactions with particular reference to variation in choice of behaviour. Anim. Behav. 1985;33:1152–1161. doi:10.1016/S0003-3472(85)80175-5 [Google Scholar]
- Enquist M, Leimar O. Evolution of fighting behavior: the effect of variation in resource value. J. Theor. Biol. 1987;127:187–205. doi:10.1016/S0022-5193(87)80130-3 [Google Scholar]
- Enquist M, Plane E, Roed J. Aggressive communication in Fulmars (Flumarus glacialis) competing for food. Anim. Behav. 1985;33:1007–1020. doi:10.1016/S0003-3472(85)80035-X [Google Scholar]
- Frey D.F, Pimental R.A. Principal component analysis and factor analysis. In: Colgan P.W, editor. Quantitative ethology. Wiley; New York, NY: 1978. pp. 219–245. [Google Scholar]
- Goubault M, Scott D, Hardy I.C.W. The importance of offspring value: maternal defence in parasitoid contests. Anim. Behav. 2007;74:437–446. doi:10.1016/j.anbehav.2006.11.029 [Google Scholar]
- Grafen A. Biological signals as handicaps. J. Theor. Biol. 1990;144:517–546. doi: 10.1016/s0022-5193(05)80088-8. [DOI] [PubMed] [Google Scholar]
- Hack M.A, Thompson D.J, Fernandes D.M. Fighting in males of the autumn spider, Metellina segmentata: effects of relative body size, prior residency and female value on contest outcome and duration. Ethology. 1997;103:488–498. [Google Scholar]
- Hansen A.J. Fighting behavior in bald eagles: a test of game theory. Ecology. 1986;67:787–797. doi:10.2307/1937701 [Google Scholar]
- Hughes M. Deception with honest signals: signal residuals and signal function in snapping shrimp. Behav. Ecol. 2000;11:614–623. doi:10.1093/beheco/11.6.614 [Google Scholar]
- Humphries E.L, Hebblethwaite A.J, Batchelor T.P, Hardy I.C.W. The importance of valuing resources: host weight and contender age as determinants of parasitoid wasp contest outcomes. Anim. Behav. 2006;72:891–898. doi:10.1016/j.anbehav.2006.02.015 [Google Scholar]
- Hurd P.L, Enquist M. A strategic taxonomy of biological communication. Anim. Behav. 2005;70:1155–1170. doi:10.1016/j.anbehav.2005.02.014 [Google Scholar]
- Jackson, N. W. 1988 Information gathering and decision making during shell selection by the hermit crab Pagurus bernhardus PhD thesis, The Queen's University of Belfast.
- Johnstone R.A. Game theory and communication. In: Dugatkin L.A, Reeve H.K, editors. Game theory and animal behaviour. Oxford University Press; New York, NY: 1998. pp. 94–117. [Google Scholar]
- Kotiaho J.S, Alatalo R.V, Mappes J, Parri S. Honesty of agonistic signalling and effects of size and motivation asymmetry in contests. Acta ethologica. 1999;2:13–21. doi:10.1007/PL00012227 [Google Scholar]
- Krebs J.R, Dawkins R. Animal signals: mind reading and manipulation. In: Krebs J.R, Davies N.B, editors. Behavioural ecology: an evolutionary approach. Blackwell; Oxford, UK: 1984. pp. 380–402. [Google Scholar]
- Lindstrom K. The effect of resource holding potential, nest size and information about resource quality on the outcome of intruder–owner conflicts in the sand goby. Behav. Ecol. Sociobiol. 1992;30:53–58. doi:10.1007/BF00168594 [Google Scholar]
- Maynard-Smith J. Game theory and the evolution of behaviour. Proc. R. Soc. B. 1979;205:475–488. doi: 10.1098/rspb.1979.0080. doi:10.1098/rspb.1979.0080 [DOI] [PubMed] [Google Scholar]
- Maynard-Smith J, Harper D. Oxford, UK; Oxford University Press: 2003. Animal signals. [Google Scholar]
- Maynard-Smith J, Parker G.A. The logic of asymmetric contests. Anim. behav. 1976;24:159–175. doi:10.1016/S0003-3472(76)80110-8 [Google Scholar]
- Neil S.J, Elwood R.W. Behavioural modification during egg-brooding in the hermit crab, Pagurus bernhardus L. J. Exp. Mar. Biol. Ecol. 1985;94:99–114. doi:10.1016/0022-0981(85)90052-8 [Google Scholar]
- Nosil P. Food fights in house crickets, Acheta domesticus, and the effects of body size and hunger level. Can. J. Zool. 2002;80:409–417. doi:10.1139/z02-018 [Google Scholar]
- Parker G.A. Assessment strategy and evolution of fighting behavior. J. Theor. Biol. 1974;47:223–243. doi: 10.1016/0022-5193(74)90111-8. doi:10.1016/0022-5193(74)90111-8 [DOI] [PubMed] [Google Scholar]
- Parker G.A, Stuart R.A. Animal behavior as a strategy optimizer: evolution of resource assessment strategies and optimal emigration thresholds. Am. Nat. 1976;110:1055–1076. doi:10.1086/283126 [Google Scholar]
- Popp J.W. Resource value and dominance among American goldfinches. Bird Behav. 1987;7:73–77. [Google Scholar]
- Prenter J, Elwood R.W, Taylor P.W. Self-assessment by males during energetically costly contests over precopula females in amphipods. Anim. Behav. 2006;72:861–868. doi:10.1016/j.anbehav.2006.01.023 [Google Scholar]
- Rand W.M, Rand A.S. Agonistic behavior in nesting iguanas: stochastic analysis of dispute settlement dominated by minimization of energy cost. Z. Tierpsychol. 1976;40:279–299. doi: 10.1111/j.1439-0310.1976.tb00938.x. [DOI] [PubMed] [Google Scholar]
- Riechert S.E. Games spiders play. II. Resource assessment strategies. Behav. Ecol. Sociobiol. 1979;6:121–128. doi:10.1007/BF00292558 [Google Scholar]
- Riechert S.E. Games spiders play. III. Cues underlying context-associated changes in agonistic behavior. Anim. Behav. 1984;32:1–15. doi:10.1016/S0003-3472(84)80318-8 [Google Scholar]
- Rodriguez-Girones M.A, Drummond H, Kacelnik A. Effect of food deprivation on dominance status in blue-footed booby (Sula nebouxii) broods. Behav. Ecol. 1996;7:82–88. doi:10.1093/beheco/7.1.82 [Google Scholar]
- Searcy W.A, Nowicki S. 1st edn. Princeton University Press; Princeton, NJ: 2005. The evolution of animal communication: reliability and deception in signaling systems. [Google Scholar]
- Sigurjonsdottir H, Parker G.A. Dung fly struggles: evidence for assessment strategy. Behav. Ecol. Sociobiol. 1981;8:219–230. doi:10.1007/BF00299834 [Google Scholar]
- Szamado S. Cheating as a mixed strategy in a simple model of aggressive communication. Anim. Behav. 2000;59:221–230. doi: 10.1006/anbe.1999.1293. doi:10.1006/anbe.1999.1293 [DOI] [PubMed] [Google Scholar]
- Taylor P.W, Elwood R.W. The mismeasure of animal contests. Anim. Behav. 2003;65:1195–1202. doi:10.1006/anbe.2003.2169 [Google Scholar]
- Verrell P.A. Wrestling in the red-spotted newt (Notophthalmus viridescens): resource value and contestant asymmetry determine contest duration and outcome. Anim. Behav. 1986;34:398–402. doi:10.1016/S0003-3472(86)80108-7 [Google Scholar]
- Zahavi A. Mate selection—a selection for a handicap. J. Theor. Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. doi:10.1016/0022-5193(75)90111-3 [DOI] [PubMed] [Google Scholar]