Abstract
Previous studies on the metabolism of capsaicinoids, natural products isolated from chili peppers, demonstrated the production of unique macrocyclic, alkyl dehydrogenated, ω-, and ω-1-hydroxylated products. This study investigated the structural and enzymatic parameters that direct selective alkyl dehydrogenation and hydroxylation of capsaicinoids, using a variety of structurally related capsaicinoid analogs and cytochrome P450 (P450) enzymes. CYP2C9 preferentially catalyzed alkyl dehydrogenation, whereas CYP2E1 and 3A4 catalyzed ω- and ω-1-hydroxylation, respectively. Analysis of incubations containing various P450s and structural variants of capsaicin by liquid chromatography-tandem mass spectrometry demonstrated similarities in the rate of capsaicinoid metabolism, but marked differences in the metabolite profiles. Production of macrocyclic and ω-1-hydroxylated metabolites from the various capsaicinoids was dependent on the structure of the alkyl terminus and P450 enzyme. A tertiary carbon at the ω-1 position, coupled to an adjacent unsaturated bond at the ω-2,3 position, enhanced the formation of the macrocyclic and dehydrogenated metabolites and were requisite structural features for ω-1-hydroxylated product formation. Conversely, substrates lacking these structural features were efficiently oxidized to the ω-hydroxylated metabolite. These data were consistent with our hypothesis that metabolism of the alkyl portion of capsaicinoids was governed, in part, by the stability and propensity to form an intermediate radical and a carbocation, and a direct interaction between the alkyl terminus and the heme of many P450 enzymes. These results provided valuable insights into potential mechanisms by which P450s metabolize capsaicinoids and highlight critical chemical features that may also govern the metabolism of structurally related compounds including fatty acids, monoterpenes, and isoprenoids.
The capsaicinoids are a family of natural products isolated from the dried fruits of chili peppers (Capsicum annum and Capsicum frutescens) (Govindarajan, 1985; Govindarajan and Sathyanarayana, 1991; Caterina et al., 1997). These substances are the principals that produce the characteristic sensations associated with the ingestion of spicy cuisine as well as the agents responsible for causing severe irritation, inflammation, erythema, and transient hyper- and hypoalgesia at sites exposed to capsaicinoids; capsaicinoids are particularly irritating to the eyes, skin, nose, tongue, and respiratory tract. There are six naturally occurring capsaicinoid analogs: capsaicin, dihydrocapsaicin, nordihydrocapsaicin, nonivamide, homocapsaicin, and homodihydro-capsaicin (Fig. 1) (Reilly et al., 2001). All capsaicinoid analogs possess a 3-hydroxy-4-methoxy-benzylamide (vanilloid ring) pharmacophore, but differ from capsaicin in their hydrophobic alkyl side chain. Differences in the side chain moiety include saturation of C15–16 (the ω-2,3 position), deletion of a methyl group at C17 (loss of the tertiary carbon), and changes in the length of the hydrocarbon chain (Fig. 1). Previous structure-activity studies using models for the study of acute pain and altered pain sensitivity in mice have demonstrated a strict structural requirement for both the vanilloid ring pharmacophore and a hydrophobic alkyl chain that may be saturated or unsaturated, branched or unbranched, and consisting of 8 to 12 carbon atoms for optimal binding and activation of the capsaicin receptor, TRPV1 (Walpole et al., 1993a,b,c).
Fig. 1.
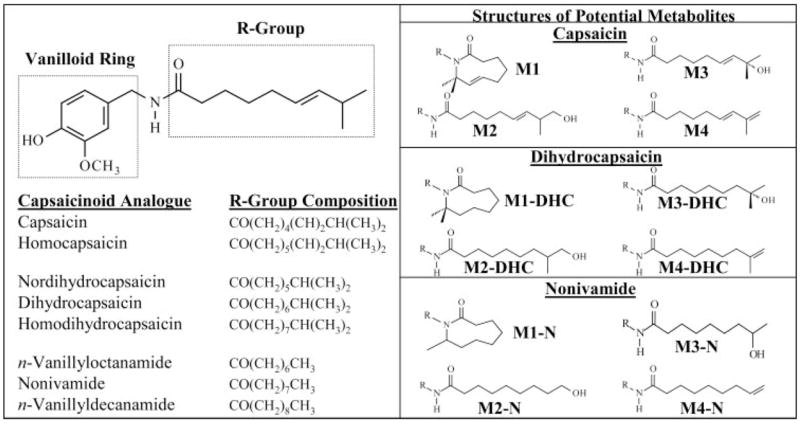
Chemical structures of the capsaicinoids and alkyl-derived metabolites of capsaicin, dihydrocapsaicin, and nonivamide. The structure of capsaicin is shown in the upper left panel. The compositions of the alkyl side chain groups (R-groups) of the capsaicinoid analogs used in this study are represented in text format below the structure of capsaicin. The right panel shows the alkyl-derived metabolites that are under investigation.
Capsaicinoids, particularly in pepper spray or over-the-counter pain relief products (e.g., Capsazin; Chattem, Inc., Chattanooga, TN), are frequently associated with undesirable effects including localized dermal erythema, tissue inflammation (e.g., skin, mucous membranes, and eyes), painful burning and itching sensations, and uncontrollable cough (Caterina et al., 1997; Olajos and Salem, 2001; NIJ, March 1994). In animals, the toxicity (LD50) of capsaicinoids has been shown to be related to the route of exposure, with intravenous and intratracheal administration being the most lethal forms of exposure, approximately 100 to 600 times more lethal than oral or topical doses (Glinsukon et al., 1980). In all cases, however, the cause of death was attributed to severe respiratory depression and cardiovascular dysfunction (Glinsukon et al., 1980). One hypothesis for the apparent tissue-selective nature of the toxicity of the capsaicinoids proposed that unique cytotoxic metabolites were produced in respiratory tissues and that these metabolites were responsible for the ensuing cellular damage, organ dysfunction, and ultimate failure. However, previous studies that characterized the P450-dependent metabolism of capsaicin demonstrated that biotransformation serves as a protective mechanism against cell death in lung and liver cells, as demonstrated by an increase in cytotoxicity when cellular P450s were inhibited by the mechanism-based inactivator, 1-aminobenzotriazole (Reilly et al., 2003a). Inhalation of pepper spray aerosols (capsaicinoids) by rats caused exposure-dependent acute tissue inflammation and selective damage to various airway epithelial cell types (Reilly et al., 2003a). Additional studies confirmed that capsaicin and its analogs produced these adverse effects through the activation of calcium-dependent (inflammation) and calcium-independent (cytotoxic) TRPV1-mediated processes (Reilly et al., 2003b). Thus, capsaicinoids have been classified as moderately toxic substances with an apparent selectivity for respiratory and cardiovascular tissues. However, the mechanisms for cellular damage and possibly organ dysfunction are not dependent upon the P450-mediated bioactivation to cytotoxicants. Rather, the toxic effects of capsaicinoids appear to be highly dependent upon the ability of capsaicin and its analogs to bind and activate TRPV1, ultimately promoting cellular inflammation and cell death.
Previous studies on the metabolism, and subsequent amelioration, of capsaicin toxicity through metabolism by P450 enzymes identified a unique alkyl-dehydrogenated metabolite (i.e., a diene of capsaicin, M4), in addition to the expected ω-, and ω-1-hydroxylated (M2 and M3, respectively) metabolites (Fig. 1). An unusual macrocyclic metabolite (M1) was also identified and was postulated to arise through covalent bond formation between the amide nitrogen and a uniquely stable tertiary allylic radical at the penultimate (ω-1) carbon of the alkyl side chain (Reilly et al., 2003a) (Fig. 1). The macrocyclic and ω-1-hydroxylated metabolites were not formed from the straight-chain capsaicin analog, nonivamide. Nonivamide lacks the tertiary allylic structural feature (Fig. 1) and, thus, does not have ability to form a stabilized radical intermediate, which was postulated to be a requisite intermediate in the production of these three metabolites.
However, our previous work did not answer many questions regarding substrate structure requirements for individual metabolite production, or elucidate whether specific cytochrome P450 enzymes imposed active site constraints that might control the formation of these unique metabolites. Could a capsaicinoid with a tertiary carbon, but without the unsaturation at the ω-2,3 position, form these types of metabolites? Could capsaicinoids with longer or shorter alkyl chains form macrocyclic products consisting of more or less carbon atoms? Would longer or shorter straight-chain analogs serve as substrates for both ω- and ω-1-hydroxylation? Therefore, the current research was conducted to determine which structural features of the capsaicinoids controlled the P450-mediated production of these alkyl-hydroxylated and -dehydrogenated metabolites, and to establish rational mechanistic hypotheses for their formation. Our working hypothesis was: the presence of a tertiary allylic carbon was crucial for the formation and stabilization of a tertiary allylic radical intermediate that ultimately produced the macrocyclic, terminal dehydrogenated, and ω-1-hydroxylated metabolites. Conversely, in the absence of this structural configuration, the alkyl chain binds to most P450s in an orientation wherein the alkyl terminus interacts directly with the P450 heme, and substrates are preferentially oxidized at the terminal methyl position to form an ω-hydroxylated metabolite.
Materials and Methods
Chemicals and Reagents
Capsaicin and its natural and synthetic analogs are potent dermal, ocular, and respiratory irritants that cause severe irritation, painful burning sensations, and uncontrollable cough. Use caution when handling concentrated solutions or powdered forms of these chemicals. 3-Hydroxy-4-methoxy-benzylamine HCl (vanillamine HCl), decanoyl chloride, octanoyl chloride, nonivamide (n-vanillylnonanamide), capsaicin (97%), capsaicin (60%), dihydrocapsaicin (90%), NADPH, sodium carbonate, sodium hydroxide, D2O, and methanol-D1 were purchased from Sigma-Aldrich (St. Louis, MO). 18O-Water (95%) was purchased from Cambridge Isotope Laboratories (Andover, MA). Anhydrous diethyl ether, n-butyl chloride, ethyl acetate, HPLC-grade methanol, and 88% formic acid were purchased from J. T. Baker (Phillipsburg, NJ). Homocapsaicin, homodihydrocapsaicin, and nordihydrocapsaicin were purified from a mixture of capsaicinoids (60% capsaicin) by HPLC. Purity values for the isolated and synthesized capsaicinoid analogs ranged from 85 to 95%. Purified water for buffer and sample preparation was generated using a Millipore Milli-Q Plus water purification system (Millipore Corporation, Billerica, MA).
Synthesis of Capsaicinoids
Synthesis of n-vanillyloctanamide and n-vanillyldecanamide was achieved using a method based on those described by Nelson (1919) and Jones and Pyman (1925). Briefly, the free-base form of vanillamine HCl was prepared by dissolving 0.2 g of vanillamine HCl in 3 ml of 0.3 N NaOH and adding 1 N NaOH (drop-wise) until a precipitate formed. The precipitate was collected by vacuum filtration, dissolved in methanol, crystallized by drying at 40°C under a stream of air, and dried at ~70–80°C for ~10 to 20 min. The molar equivalents of solid vanillamine were determined, and the powder was mixed with 5 ml of anhydrous ethyl ether to produce a suspension. The appropriate acyl chloride was added to this suspension in a drop-wise manner to a total molar ratio of 0.5:1. The mixture was gently heated in a water bath, removed, capped, and mixed. The mixture was then permitted to react overnight at room temperature (~22°C) with gentle rocking. The next day, 50 μl of 1 M HCl and 2 ml of H2O were added and mixed to remove the excess, unreacted vanillamine. The ether fraction was transferred and washed with 2 ml of 50% saturated sodium carbonate to decompose the remaining acyl chloride. The ether fraction was collected and dried under a stream of air at 40°C, and the product was dissolved in methanol. The molecular mass of the two capsaicinoid products was confirmed by direct infusion of a 10 ng/μl solution of each of the products (in methanol/0.1% formic acid, 70:30) into the mass spectrometer, and were verified as m/z 280 and 308 for the octyl and decyl forms, respectively. The structural features were determined using tandem mass spectrometry and comparison to the mass spectra of other capsaicinoid standards. Purity and retention properties were assessed by HPLC and UV detection at 230 nm, using nonivamide as the quantitative standard. Products were ~90 to 95% pure with an approximate yield of 25 to 30%.
In Vitro Metabolism
The metabolism of capsaicin and its chemical analogs (see Fig. 1 for structures) was performed, as previously described (Reilly et al., 2003a), using pooled human liver microsomes (100 pmol/ml, ~0.25 mg/ml) and various recombinant human P450 enzymes (50 pmol/ml) (BD Gentest, Woburn, MA). Briefly, 100 μM capsaicinoid was incubated with each P450 sample and 2 mM NADPH in phosphate-buffered saline (pH 7.2) for various time points up to 1 h at 37°C (0.5 ml, total volume); 100 μM capsaicin appeared to be a saturating concentration for these enzymes. The metabolites were extracted from the incubations using 4 ml of 50% n-butyl chloride/50% ethyl acetate, fortified with 50 μM capsaicin (or nonivamide) as an internal standard (based on a 500-μl incubation volume), mixed, and centrifuged; and the organic layer was collected and concentrated by drying at 40°C under a stream of air. Prior to LC/MS/MS analysis, the dried residues were reconstituted in 50 μl of 60% methanol/40% purified H2O.
Analysis of Metabolite Formation by Liquid Chromatography-Tandem Mass Spectrometry
Electrospray-ionization LC/MS/MS analysis was performed using a Finnegan TSQ 7000 tandem mass spectrometer Thermo Electron (Waltham, MA) interfaced with a Hewlett Packard Series 1100 solvent delivery system (Hewlett Packard, Palo Alto, CA). The metabolites were resolved using a MetaSil Basic C2-C6 (150 × 3.0, 3 μm) reverse-phase HPLC column (Varian, Inc., Palo Alto, CA) with isocratic conditions consisting of methanol and aqueous formic acid (0.1% v/v). The chromatographic conditions used for the analysis of each analog and its metabolites were as follows: n-vanillyloctanamide (57.5% methanol); nordihydrocapsaicin, capsaicin, and nonivamide (60% methanol); dihydrocapsaicin and n-vanillyldecanamide (64% methanol); and homocapsaicin and homodihydrocapsaicin (68%). These concentrations were chosen to produce overlapping retention times for the individual analogs and their respective metabolites, relative to capsaicin. The HPLC flow rate was set at 0.25 ml/min and the column was maintained at a temperature of 40°C. The mass spectrometer was programmed to monitor for precursor-to-product ion transitions corresponding to the internal standard (capsaicin or nonivamide), parent compounds ([M + H]+), dehydrogenated ([M + H + 2 amu]+), and oxygenated ([M + H + 16 amu]+) metabolites, and the primary capsaicinoid product ion derived from the vanilloid ring moiety (m/z 137). The parent compound m/z ratios were 280 (n-vanillyloctanamide), 294 (nonivamide and nordihydrocapsaicin), 306 (capsaicin), 308 (dihydrocapsaicin, n-vanillyldecanamide), 320 (homocapsaicin), and 322 (homodihydro-capsaicin). The identity of each capsaicinoid metabolite was verified by analysis in 60% D2O/methanol-D1 and by full-scan MS/MS analysis, as previously described for capsaicin (Reilly et al., 2003a). Incorporation of 18O from 18O-Water (50% and 95% v/v in incubations) was assessed by full-scan mass spectrometry and monitoring for increases in the +2 amu isotope peak. The parameters for the mass spectrometer were optimized using nonivamide and the “optimize” function within the instrument operating system. All other parameters were as follows: collision gas (argon), 3.0 mT; collision-offset voltage, −20 eV; auxiliary gas (nitrogen), 10 units; and sheath gas (nitrogen), 50 psi.
Relative metabolite production was determined by dividing the normalized metabolite peak area ratios by the corresponding ratios obtained for capsaicin. Semiquantitative analysis of metabolite production was assessed by integration of the selected metabolite peaks in the LC/MS/MS chromatogram and normalizing the peak area to the internal standard peak area (capsaicin or nonivamide). Absolute quantitation of the metabolites was not feasible because analytical standards are not available. Quantitative analysis to assess metabolic rates for capsaicin and nonivamide was achieved by monitoring the disappearance of the substrate and determining the change in substrate concentration using peak area ratios (analyte/internal standard) and a standard curve constructed with the specific capsaicinoid analog.
Results
Analysis of capsaicin, its analogs, and the production of their respective metabolites was achieved using the analytical methods described above. The rates and extent of capsaicin and nonivamide metabolism by human liver microsomes were identical and quickly (< 10 min) became nonlinear during the 60-min incubation period (Fig. 2A). The initial rate of substrate disappearance due to P450 turnover in human liver microsomes was 4 ± 1 pmol/min · pmol P450 for both capsaicin and nonivamide. Production of the alkyl-hydroxylated (ω-, ω-1-) and dehydrogenated (macrocyclic and terminal alkene) metabolites (M1-M4) from capsaicin and nonivamide (M1-M4-N) was also nonlinear (Fig. 2, B and C). The rates of formation and final concentrations of the four alkyl-derived capsaicin metabolites were different. Surprisingly, the dehydrogenated alkene, M4, was produced at the fastest rate and highest final concentration. M3 was the least abundant metabolite produced, whereas M1 and M2 were similar in their rate of formation and abundance (Fig. 2B). The relative production of each metabolite from capsaicin is presented in Table 1.
Fig. 2.
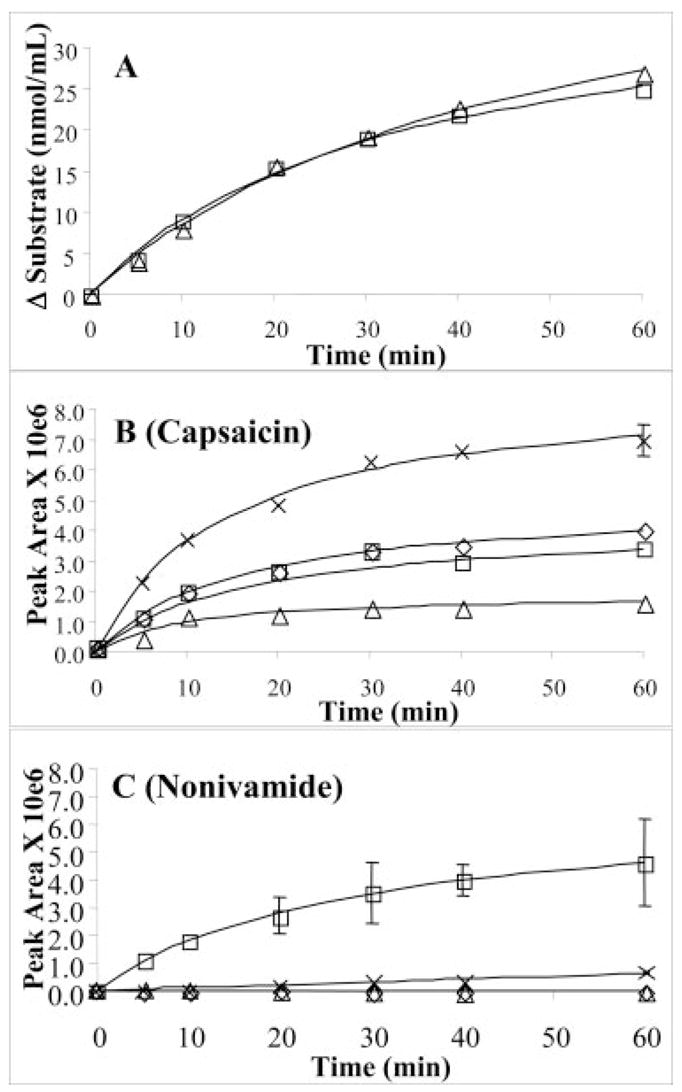
Quantitative kinetic analysis of the metabolism of capsaicin (open triangles) and nonivamide (open squares) by human liver microsomes (A). Semiquantitative kinetic data representing the relative formation of macrocyclic (open diamonds), ω-hydroxylated (open squares), ω-1-hydroxylated (open triangles), and terminal dehydrogenated (crosses) metabolites from capsaicin (B) and nonivamide (C) by human liver microsomes. Incubations were prepared and assayed as described under Materials and Methods using either capsaicin or nonivamide as an internal standard (i.e., nonivamide was used as the internal standard for incubations containing capsaicin). Data are representative of the mean relative metabolite peak area and standard deviation for three separate incubations at each time point. For clarity, error bars < 7% have been omitted from the figure; these error bars were encompassed by the symbol.
TABLE 1. Relative metabolite abundance produced from the metabolism of capsaicin and nonivamide by human liver microsomes and recombinant human CYP2C9.
Incubations were performed as described under Materials and Methods. Relative metabolite abundance was determined as the percentage of total metabolite peak area obtained from selected ion monitoring LC/MS for M1 to M9 (n = 3) (Reilly et al., 2003a).
| HLM
|
CYP2C9
|
|||
|---|---|---|---|---|
| Metabolite Identification | Capsaicin | Nonivamide | Capsaicin | Nonivamide |
| % of total metabolites | % of total metabolites | |||
| Macrocycle (M1) | 22 ± 4 | N.D. | 34 ± 3 | N.D. |
| ω-OH (M2) | 18 ± 4 | 24 ± 4 | 1 ± 0.2 | 0.8 ± 0.1 |
| ω-1-OH (M3) | 8 ± 2 | N.D. | < 0.1 | N.D. |
| Diene or alkene (M4) | 28 ± 5 | 4 ± 1 | 63 ± 5 | 5 ± 2 |
| Others (M5–M9) | 28 ± 14 | 71 ± 4 | < 2 | 95 ± 4 |
HLM, human liver microsomes; N.D., not detected.
The production of alkyl-derived metabolites from nonivamide was strikingly different from that for capsaicin (Fig. 2C). Specifically, the putative macrocyclic (M1-N) and ω-1-hydroxylated (M3-N) metabolites were not detected, and the production of the dehydrogenated product, M4-N, was minimal. The ω-hydroxylated metabolite, M2-N, was the principal product of this straight-chain capsaicinoid. Interestingly, the formation of M4-N was not observed until the 20-min time point, suggesting that either the rate of M4-N production by P450 was minimal and product accumulation was not detectable until 20 min, or M4-N was produced via a secondary, perhaps nonenzymatic process such as dehydration of M2-N. Regardless, significant differences in the production of the alkyl-hydroxylated and -dehydrogenated metabolites were observed for these structurally diverse capsaicinoid analogs.
Incubations with capsaicin and a variety of recombinant P450 enzymes also demonstrated significant differences in metabolite production (Fig. 3). CYP2C9, 2C19, 2C8, and 2E1 efficiently catalyzed the formation of the dehydrogenated metabolites M1 and M4 from capsaicin. However, CYP2E1 produced M2 with the highest relative turnover, and M3 production was highest with CYP3A4 and 2C8. Additional P450 enzymes that produced metabolites included CYP1A1, 1A2, 2D6, and 2A6; however, their catalytic efficiency was markedly lower than that of human liver microsomes or the enzymes discussed previously. Because CYP2C9 was the most active enzyme for catalyzing the alkyl dehydrogenation of capsaicin to form M1 and M4, coupled with the low amounts of M2 and M3 formed, it was chosen for additional studies to identify similar metabolites from a variety of structurally diverse capsaicinoid analogs.
Fig. 3.
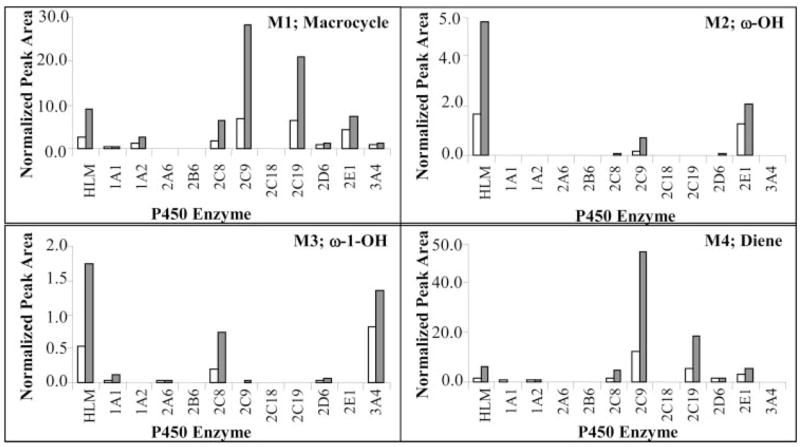
Formation of M1, M2, M3, and M4 from capsaicin by individual recombinant P450 enzymes and human liver microsomes (HLM). Note that the y-axis for M4 is approximately 2-, 10-, and 30-fold higher than the relative responses for M1, M2, and M3. Incubations containing individual P450 enzymes were prepared and assayed as described under Materials and Methods using nonivamide as an internal standard. Data represent the normalized metabolite peak area (analyte/internal standard) from individual samples that were incubated for 20 (open bars, n = 1) or 60 min (gray bars, n = 1).
Data showing the relative production of metabolites corresponding to M1-M4 (from capsaicin) by human liver microsomes and CYP2C9 from structural analogs of capsaicin (see Fig. 1), are shown in Tables 2 and 3, respectively. The production of macrocyclic metabolites was limited to those capsaicinoid analogs that contained a tertiary carbon atom at the ω-1 position. In addition, the production of these metabolites was markedly enhanced by the presence of a double bond at the ω-2,3 position (i.e., capsaicin and homocapsaicin). Small amounts (< 7% of capsaicin) of M1-like metabolites were observed with the saturated capsaicin analogs nordihydrocapsaicin, dihydrocapsaicin, and homodihydrocapsaicin; formation of macrocyclic metabolites from these analogs was verified by deuterium exchange MS/MS analysis and the presence of a single exchangeable proton (Reilly et al., 2003a). Significant production of M1 from capsaicin and its corresponding macrocyclic metabolite from homocapsaicin was also observed. Formation of macrocyclic metabolites was not observed when the straight-chain analogs of capsaicin (i.e., n-vanillyloctanamide, nonivamide, and n-vanillyldecanamide) were the substrates. ω-Hydroxylated metabolites were produced from all of the capsaicinoid analogs. However, significantly greater amounts of these metabolites were observed when straight-chain or branched-chain saturated capsaicinoid analogs were the substrates.
TABLE 2. Production of metabolites related to M1, M2, M3, and M4 (from capsaicin) from various capsaicinoid analogs by human liver microsomes.
Values represent the average percentage and standard deviation for metabolite production, relative to capsaicin, from three separate incubations. Incubations were performed as described under Materials and Methods using the semiquantitative method for assessing metabolite formation.
| Capsaicinoid Analog and Description | Relative Metabolite Production Versus Capsaicin | |||
|---|---|---|---|---|
| % | ||||
| Saturated analogs | Macrocycle | ω-OH | ω-1-OH | Terminal alkene |
| Nordihydrocapsaicin | 3.3 ± 0.9 | 90 ± 14 | N.D. | 15 ± 4 |
| Dihydrocapsaicin | 6 ± 2 | 180 ± 15 | N.D. | 23 ± 9 |
| Homodihydrocapsaicin | 10 ± 2 | 193 ± 7 | N.D. | 29 ± 6 |
| Straight-chain analogs | Macrocycle | ω-OH | ω-1-OH | Terminal alkene |
| n-Vanillyloctanamide | N.D. | 54 ± 5 | N.D. | 3 ± 1 |
| Nonivamide | N.D. | 135 ± 8 | N.D. | 6 ± 3 |
| n-Vanillyldecanamide | N.D. | 170 ± 15 | N.D. | 14 ± 5 |
| Increased chain lengths | Macrocycle | ω-OH | ω-1-OH | Diene |
| Homocapsaicin | 48 ± 8 | 50 ± 13 | 60 ± 12 | 8 ± 1 |
N.D., not detected.
TABLE 3. Production of metabolites related to M1, M2, M3, and M4 (from capsaicin) from various capsaicinoid analogs by recombinant human CYP2C9.
Values represent the average percentage of metabolite produced, relative to capsaicin, from two separate incubations. Incubations were performed as described under Materials and Methods using the semiquantitative method for assessing metabolite formation.
| Capsaicinoid Analog and Description | Relative Metabolite Production Versus Capsaicin | |||
|---|---|---|---|---|
| % | ||||
| Saturated analogs | Macrocycle | ω-OH | ω-1-OH | Terminal alkene |
| Nordihydrocapsaicin | 0.9 | 103 | N.D. | 6.6 |
| Dihydrocapsaicin | 4.5 | 349 | N.D. | 40 |
| Homodihydrocapsaicin | 4.4 | 285 | N.D. | 26 |
| Straight chain analogs | Macrocycle | ω-OH | ω-1-OH | Terminal alkene |
| n-Vanillyloctanamide | N.D. | 30 | N.D. | 1.1 |
| Nonivamide | N.D. | 267 | N.D. | 17 |
| n-Vanillyldecanamide | N.D. | 247 | N.D. | 8.8 |
| Increased chain lengths | Macrocycle | ω-OH | ω-1-OH | Diene |
| Homocapsaicin | 24 | 106 | 64 | 5.3 |
N.D., not detected.
Similar to the macrocyclic metabolites, significant quantities of ω-1-hydroxylated metabolites were only produced from capsaicin and homocapsaicin. The terminal dehydrogenated metabolites were produced from all of the capsaicinoid analogs, with the rank order for production of: analogs with a tertiary allylic carbon > analogs with a tertiary carbon only > straight-chain analogs (Table 2). Similar data for metabolite production were observed when CYP2C9 was utilized instead of human liver microsomes, albeit produced at different absolute and relative amounts (Table 3).
Discussion
The ability of cytochrome P450 enzymes to metabolize capsaicinoids to a number of distinct metabolites has been shown to be an important determinant of the pharmacology and toxicology of these compounds. P450-dependent metabolism of capsaicinoids has been shown to mitigate capsaicinoid toxicity in cell culture (Reilly et al., 2003a). Likewise, metabolites of capsaicin did not prolong phenobarbital-induced sleep, as observed for the parent capsaicinoids (Surh et al., 1995). Structure-activity studies with capsaicin, nonivamide, structural variants, and their metabolites, have also demonstrated marked differences in pain-producing potential and hyper- and hypoalgesic properties. Chemical modifications to the alkyl terminus substantially decreased biological responses, presumably due to decreased interactions between the metabolites and TRPV1 (Walpole et al., 1993a,b,c).
The biological activities of capsaicinoids are distinctly reliant on the chemical structure of the alkyl terminus. Likewise, structural variants of capsaicin, in the form of multiple capsaicinoid analogs, exhibit unique metabolite profiles and provide an interesting set of probes to investigate aliphatic hydroxylation and dehydrogenation mechanisms by P450 enzymes. Incubations containing capsaicin or nonivamide exhibited similarities in the overall rate of metabolism by human liver microsomes (Fig. 2A). However, significant differences in the formation of alkyl-derived metabolites were observed (Fig. 2, B and C). Specifically, macrocyclic and ω-1-hydroxylated metabolites were not produced from nonivamide, and the terminal dehydrogenated metabolite of nonivamide (M4-N) was formed in very low amounts, relative to capsaicin. Similar data were observed using a variety of structurally diverse capsaicinoid analogs (Fig. 1; Tables 2 and 3), which also illustrated a critical role for branched and/or unsaturated alkyl termini for selected metabolite formation.
Hydroxylation and dehydrogenation of alkanes via the formation of ω-1 radicals and subsequent hydroxyl rebound to generate the corresponding alcohol product is a widely accepted P450 mechanism that has been described for a number of P450-catalyzed reactions (Rettie et al., 1988; Obach, 2001; Kumar et al., 2004; Meunier et al., 2004). However, the formation of ω-hydroxylated products is typically limited by unfavorable energetic barriers for substrate activation at the terminal position, unless the reactions are sterically directed to the terminal carbon within the P450 active site. Although our data support a mechanism of hydroxyl rebound and sterically controlled activation of the alkyl terminus for the formation of ω-hydroxylated metabolites from capsaicinoids, this mechanism does not fully account for the product profiles observed for all of the analogs. Therefore, we present an alternate mechanism (Fig. 4) to explain our data. This mechanism proposes that capsaicinoid metabolism proceeds through an initial hydrogen atom abstraction from the terminal carbon (sterically directed substrate activation) by the highvalent [Fe]V=O species, followed by either oxygen rebound to produce ω-alcohols from [Fe]IV–OH, or one-electron oxidation by [Fe]IV–OH to form carbocation intermediates that rearrange to the more energetically stable ω-1 tertiary carbon atoms of certain capsaicinoid analogs. These stabilized tertiary carbocation intermediates then undergo one of three reactions (Kumar et al., 2004; Meunier et al., 2004): 1) lose a proton to produce terminal alkenes, 2) become trapped by the amide nitrogen to form macrocyclic metabolites, or 3) undergo oxygen rebound from the reduced [Fe]III–OH heme to produce ω-1 alcohols (Fig. 4, A to C). We concluded that the latter reaction most likely occurred as a result of oxygen rebound from the heme rather than hydration of the carbocation by H2O, since we did not observe 18O-labeling of the hydroxylated metabolites when 18O-Water was included at 50 or 90% (v/v) in incubations (data not shown). The relative amounts of dehydrogenated and hydroxylated products produced from the various capsaicinoid analogs support this alternate mechanism in which the relative stability of the rearranged intermediate carbocation governed the relative production of ω-, ω-1, macrocyclic, and terminal dehydrogenated metabolites.
Fig. 4.
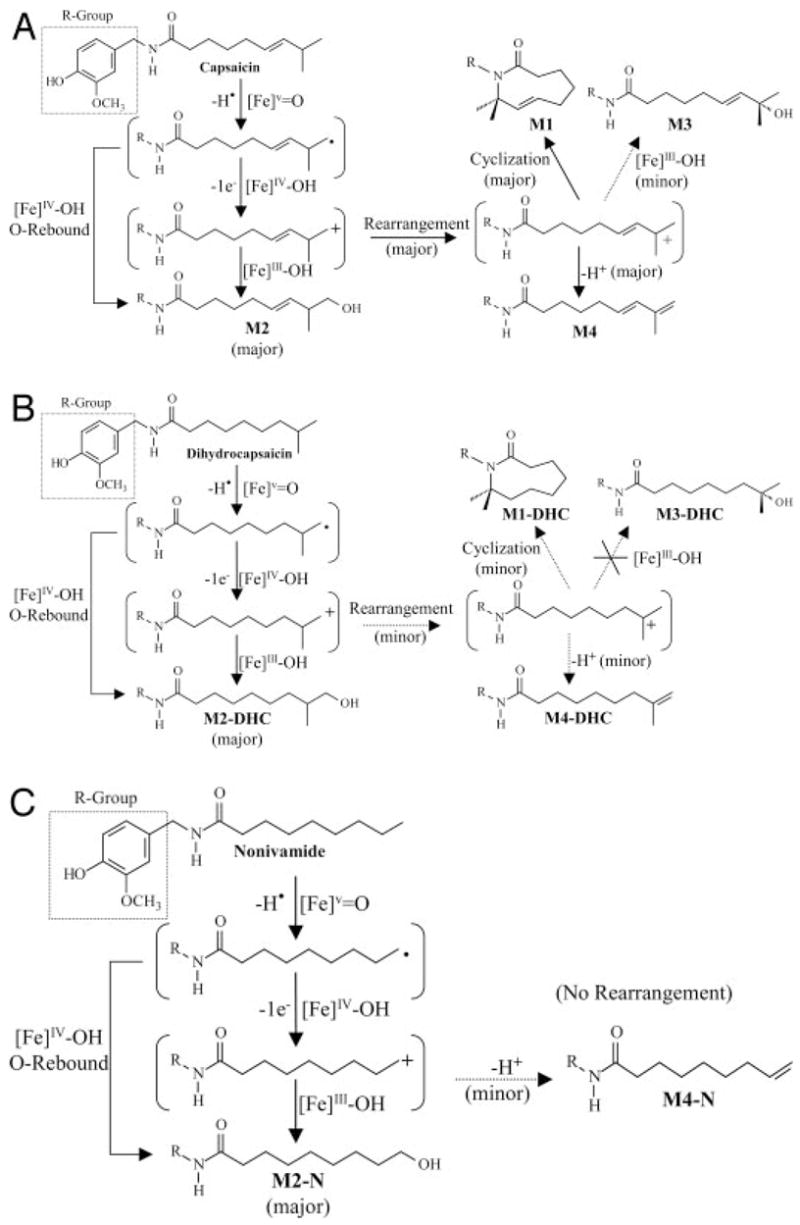
Proposed metabolic schemes describing the production of macrocyclic, ω-hydroxylated, ω-1-hydroxylated, and terminal dehydrogenated metabolites from various capsaicinoid analogs by P450 enzymes. Metabolic scheme for the metabolism of capsaicin (A), and metabolic scheme for the metabolism of dihydrocapsaicin (B) and nonivamide (C).
Preferential activation of the terminal methyl position appeared to be favored for many P450 enzymes despite the higher energetic barriers associated with activation of the primary carbon atom. Terminal activation was due presumably to steric restrictions that limit the access and orientation of the substrate relative to the active site ironoxo high-valent heme. Preferential oxidation of the terminal carbon versus ω-1-oxidation is not uncommon but is less favorable, as alluded to above. Here we provide evidence for direct activation of the terminal methyl carbon and subsequent formation of ω-hydroxylated alcohols, a process that appears to be inversely related to the predicted stability of the rearranged product (i.e., straight-chain analogs form greater amounts of ω-hydroxylated products than do saturated capsaicinoids and capsaicinoids with a tertiary allylic motif, due, presumably, to lower propensities for rearrangement to secondary carbocations that are not highly stabilized, like the tertiary allylic carbocation of capsaicin). Deuterium isotope effects in the formation of 5-hydroxy valproic acid by CYP4B1 (Rettie et al., 1995) and the formation of 20-hydroxyeicosatetraenoic acid by 4F2 and 4A11 (Powell et al., 1998) also support the concept of sterically controlled terminal hydrogen abstraction.
Formation of the respective ω-1-hydroxylated metabolites from the various capsaicinoid analogs also exhibited a strict requirement for the tertiary allylic configuration. Surprisingly, no secondary or tertiary alcohols were produced from the straight-chain or branched-chain, saturated capsaicinoids (Tables 2 and 3). These data, along with other aspects of the observed product profiles, suggest that the formation of the ω-1-alcohols from capsaicin and homocapsaicin was also accomplished through formation of a terminal methyl carbocation, rapid rearrangement of the primary carbocation to the more stable tertiary allylic carbocation, and facile oxygen rebound (minor pathway). This mechanism was consistent with the finding that incorporation of 18O-labeled water was not observed, and no secondary alcohols were formed from straight-chain capsaicinoids, despite the observation that terminal alkenes were formed (albeit in very small amounts) from these analogs. This mechanism also explains why the analogs with a tertiary carbon, but without a double bond (i.e., nordihydrocapsaicin, dihydrocapsaicin, and homodihydrocapsaicin) were not metabolized to produce ω-1-alcohols, but did produce small amounts of their respective macrocyclic metabolites and large amounts of their respective ω-hydroxylated metabolites.
Terminal dehydrogenated metabolites (M4) could potentially form by dehydration of the hydroxylated products. However, several results confirmed that dehydrogenation was directly catalyzed by P450 enzymes and not formed by dehydration of the alcohols. Specifically, the production of terminal dehydrogenated metabolites from straight-chain analogs was very low, despite extensive formation of ω-hydroxylated metabolites that surpassed that of capsaicin. Comparable results were observed for branched chain metabolites lacking the double bond at the ω-2,3 position. If M4 was formed by chemical dehydration of either the ω- or ω-1-alcohol, much greater amounts of the alkene should have been produced from these analogs, similar to the relative formation of M4 versus M2 or M3 from capsaicin. Thus, terminal dehydrogenation appeared to be a direct P450-catalyzed process, similar to that which has been shown for valproic acid, another saturated alkane, and ezlopitant (Rettie et al., 1988, 1995; Obach, 2001).
As with most P450-catalyzed processes, the ability of specific enzymes to catalyze certain metabolic reactions can be highly variable and specific. Several P450 enzymes selectively catalyzed the alkyl hydroxylation and dehydrogenation of capsaicin (Fig. 3). Based on these data, the relative percentages of individual P450 enzymes in liver microsomes, and data presented in Figs. 2, B and C, and 3, it may be reasonable to conclude that the most active enzymes for production of specific metabolites ultimately dictate the formation of these metabolites in microsomes. For example, CYP2C9 (~20% of total liver microsomal P450) (Guengerich, 2003) was likely the enzyme that produced the majority of the macrocyclic and alkene products observed in microsomal incubations, since it was the most active enzyme, and enzymes such as CYP2C8, 2C19, and 2E1 (~20% total P450, combined) produced only small quantities of these metabolites. Similar conclusions may be made regarding ω-hydroxylated metabolites by CYP2E1 and ω-1-hydroxylated products by CYP3A4. Based on the mechanistic conclusions discussed above, and those represented by Fig. 4, A to C, several general conclusions can be made regarding the underlying biochemistry for the enzyme-selective production of the metabolites. Depending upon the specific active site orientation of the capsaicinoids within the individual P450s, the relative production of metabolites will vary. For example, CYP2C9 was capable of producing all four alkyl metabolites in various amounts, with a distinct preference for catalyzing the metabolism of capsaicin to M1 and M4. These were the major products of CYP2C9 metabolism (Fig. 3; Table 1) and appeared to be produced via the mechanisms discussed above. Likewise, CYP1A2 appeared to be unique in its ability to only produce the alkyl-dehydrogenated metabolites, albeit at very low amounts, suggesting an unusual mechanism similar to that of CYP2C9, where dehydrogenation of the alkyl chain was more favorable than hydroxylation. Conversely, CYP3A4 produced large amounts of the ω-1-alcohol (Fig. 3). This difference in relative metabolite production may be due to the fact that CYP3A4 has a relatively spacious active site relative to other P450s and, therefore, may be the most likely enzyme to permit the binding of capsaicin in a manner that favors the initial abstraction of hydrogen and one-electron oxidation to produce the energetically more favorable tertiary allylic carbocation, rather than formation of this intermediate by rearrangement of the terminal carbocation. Despite the possibility that capsaicinoids were initially activated by CYP3A4 at the ω-1 position to produce M3, very little M1 or M4 was observed. These data indicate that specific interactions between individual P450 enzyme and capsaicinoids work in concert with the structural-dependent mechanisms described in Fig. 4, A to C, to direct the ultimate metabolite profile.
Overall, the metabolism of capsaicin and its analogs by human P450 enzymes presents unique insights into possible mechanisms by which P450s metabolize various chemicals and exemplify a number of theoretical aspects of P450 metabolism. The metabolism of capsaicinoids provides valuable information on chemical features that can influence the hydroxylation and dehydrogenation of certain chemical entities as well as highlight the importance of steric interactions in governing metabolite formation.
Acknowledgments
We thank the Center for Human Toxicology at the University of Utah for access to LC/MS/MS instrumentation and Dr. Paul Ortiz de Montellano for insightful comments regarding metabolic mechanisms.
This work was supported by grants from the National Heart, Lung, and Blood Institute (HL069813 and HL13645) and a contract from the National Institute of Standards and Technology (Department of Commerce Contract 60NAN-BOD0006).
ABBREVIATIONS
- TRPV1
capsaicin receptor transient receptor potential V1
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- P450
cytochrome P450
- HPLC
high-performance liquid chromatography
- LC/MS/MS
liquid chromatography-tandem mass spectrometry
- amu
atomic mass unit(s)
References
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature (Lond) 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Glinsukon T, Stitmunnaithum V, Toskulkao C, Buranawuti T, Tangkrisanavinont V. Acute toxicity of capsaicin in several animal species. Toxicon. 1980;18:215–220. doi: 10.1016/0041-0101(80)90076-8. [DOI] [PubMed] [Google Scholar]
- Govindarajan VS. Capsicum production, technology, chemistry, and quality. Part 1: History, botany, cultivation and primary processing. Crit Rev Food Sci Nutr. 1985;22:109–176. doi: 10.1080/10408398509527412. [DOI] [PubMed] [Google Scholar]
- Govindarajan VS, Sathyanarayana MN. Capsicum production, technology, chemistry, and quality. Part V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain and desensitization sequences. Crit Rev Food Sci Nutr. 1991;29:435–474. doi: 10.1080/10408399109527536. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Cytochromes P450, drugs and diseases. Mol Intervent. 2003;3:194–204. doi: 10.1124/mi.3.4.194. [DOI] [PubMed] [Google Scholar]
- Jones ECS, Pyman FL. Relation between chemical composition and pungency in acid amides. J Am Chem Soc. 1925;127 [Google Scholar]
- Kumar D, De Visser SP, Shaik S. Oxygen economy of cytochrome P450: what is the origin of the mixed functionality as a dehydrogenaseoxidase enzyme compared with its normal function? J Am Chem Soc. 2004;126:5072–5073. doi: 10.1021/ja0318737. [DOI] [PubMed] [Google Scholar]
- Meunier B, de Visser SP, Shaik S. Mechanism of oxidation reactions catalyzed by cytochrome p450 enzymes. Chem Rev. 2004;104:3947–3980. doi: 10.1021/cr020443g. [DOI] [PubMed] [Google Scholar]
- Nelson E. Vanillyl-acyl amides. J Am Chem Soc. 1919;41:2121–2130. [Google Scholar]
- [NIJ] National Institute of Justice. National Institute of Justice Technology Assessment Program. U.S. Department of Justice, Office of Justice Programs, National Institute of Justice; Washington, D.C: Mar, 1994. Oleoresin capsicum: pepper spray as a force alternative. [Google Scholar]
- Obach RS. Mechanism of cytochrome P4503A4- and 2D6-catalyzed dehydrogenation of ezlopitant as probed with isotope effects using five deuterated analogs. Drug Metab Dispos. 2001;29:1599–1607. [PubMed] [Google Scholar]
- Olajos EJ, Salem H. Riot control agents: pharmacology, toxicology, biochemistry and chemistry. J Appl Toxicol. 2001;21:355–391. doi: 10.1002/jat.767. [DOI] [PubMed] [Google Scholar]
- Powell PK, Wolf I, Jin R, Lasker JM. Metabolism of arachidonic acid to 20-hydroxy-5,8,11,14-eicosatetraenoic acid by P450 enzymes in human liver: involvement of CYP4F2 and CYP4A11. J Pharmacol Exp Ther. 1998;285:1327–1336. [PubMed] [Google Scholar]
- Reilly CA, Crouch DJ, Yost GS. Quantitative analysis of capsaicinoids in fresh peppers, oleoresin capsicum and pepper spray products. J Forensic Sci. 2001;46:502–509. [PubMed] [Google Scholar]
- Reilly CA, Ehlhardt WJ, Jackson DA, Kulanthaivel P, Mutlib AE, Espina RJ, Moody DE, Crouch DJ, Yost GS. Metabolism of capsaicin by cytochrome P450 produces novel dehydrogenated metabolites and decreases cytotoxicity to lung and liver cells. Chem Res Toxicol. 2003a;16:336–349. doi: 10.1021/tx025599q. [DOI] [PubMed] [Google Scholar]
- Reilly CA, Taylor JL, Lanza DL, Carr BA, Crouch DJ, Yost GS. Capsaicinoids Cause Inflammation and Epithelial Cell Death through Activation of Vanilloid Receptors. Toxicol Sci. 2003b;73:170–181. doi: 10.1093/toxsci/kfg044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettie AE, Boberg M, Rettenmeier AW, Baillie TA. Cytochrome P-450-catalyzed desaturation of valproic acid in vitro. Species differences, induction effects and mechanistic studies. J Biol Chem. 1988;263:13733–13738. [PubMed] [Google Scholar]
- Rettie AE, Sheffels PR, Korzekwa KR, Gonzalez FJ, Philpot RM, Baillie TA. CYP4 isozyme specificity and the relationship between omega-hydroxylation and terminal desaturation of valproic acid. Biochemistry. 1995;34:7889–7895. doi: 10.1021/bi00024a013. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Ahn SH, Kim KC, Park JB, Sohn YW, Lee SS. Metabolism of capsaicinoids: evidence for aliphatic hydroxylation and its pharmacological implications. Life Sci. 1995;56:PL305–PL311. doi: 10.1016/0024-3205(95)00091-7. [DOI] [PubMed] [Google Scholar]
- Walpole CS, Wrigglesworth R, Bevan S, Campbell EA, Dray A, James IF, Masdin KJ, Perkins MN, Winter J. Analogues of capsaicin with agonist activity as novel analgesic agents; structure-activity studies. 2. The amide bond “B-region”. J Med Chem. 1993a;36:2373–2380. doi: 10.1021/jm00068a015. [DOI] [PubMed] [Google Scholar]
- Walpole CS, Wrigglesworth R, Bevan S, Campbell EA, Dray A, James IF, Masdin KJ, Perkins MN, Winter J. Analogues of capsaicin with agonist activity as novel analgesic agents; structure-activity studies. 3. The hydrophobic side-chain “C-region”. J Med Chem. 1993b;36:2381–2389. doi: 10.1021/jm00068a016. [DOI] [PubMed] [Google Scholar]
- Walpole CS, Wrigglesworth R, Bevan S, Campbell EA, Dray A, James IF, Perkins MN, Reid DJ, Winter J. Analogues of capsaicin with agonist activity as novel analgesic agents; structure-activity studies. 1. The aromatic “A-region”. J Med Chem. 1993c;36:2362–2372. doi: 10.1021/jm00068a014. [DOI] [PubMed] [Google Scholar]


