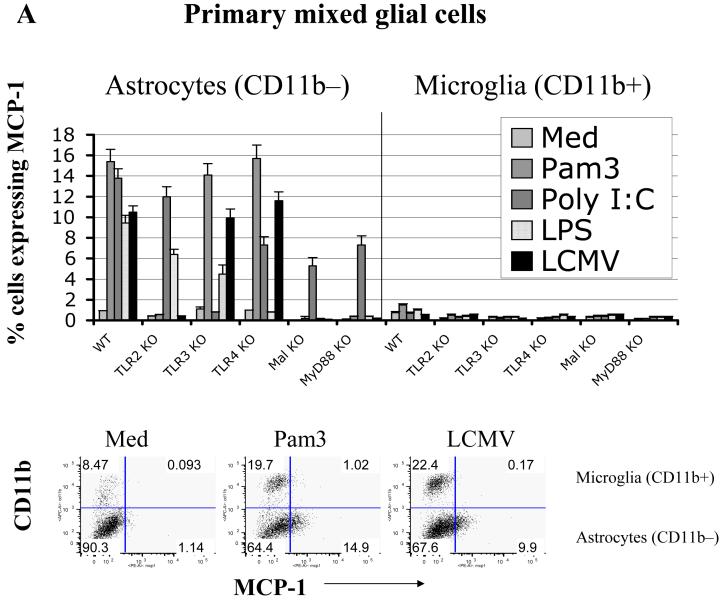
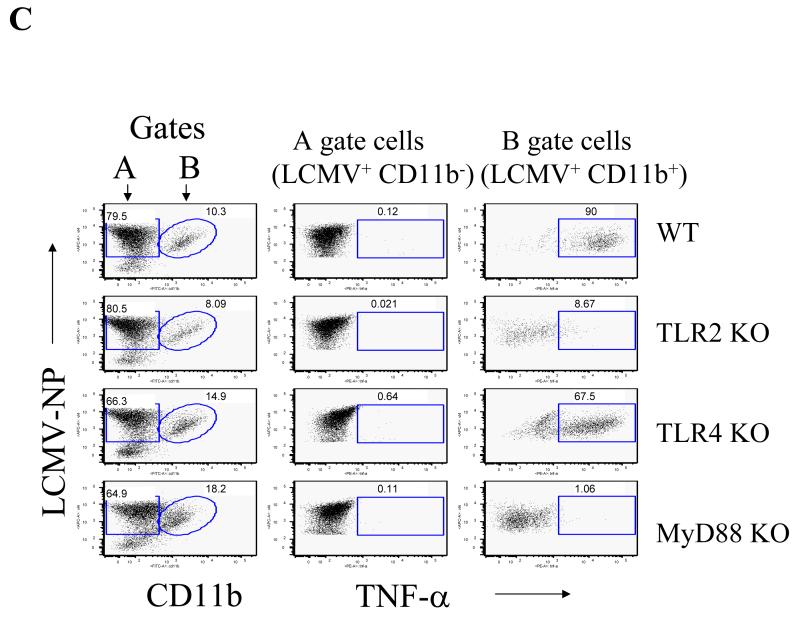
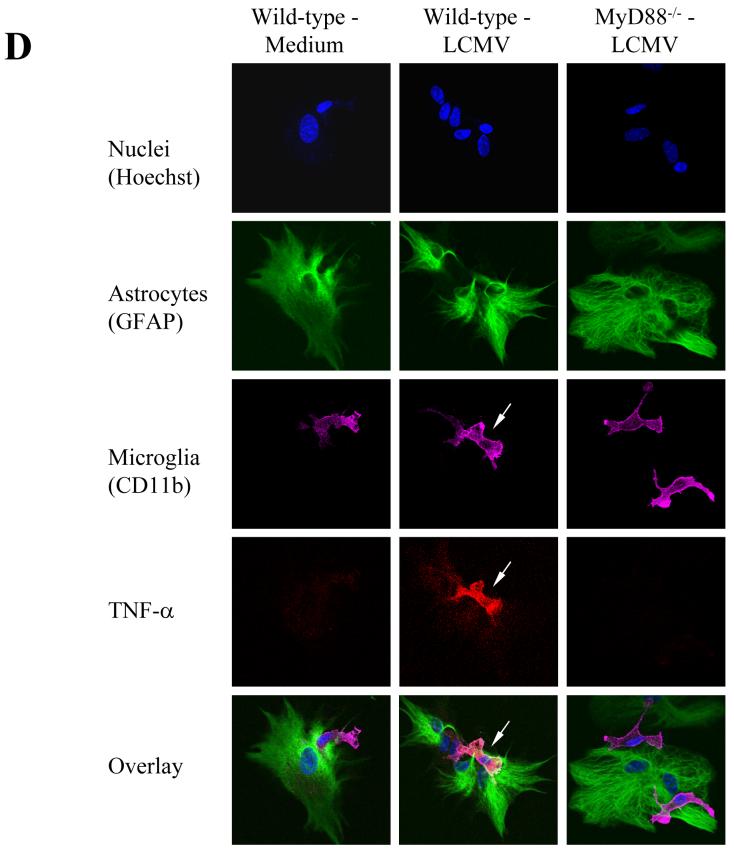
Fig 2. In response to PAMPs, including LCMV, primary astrocytes primarily produce MCP-1, whereas primary microglial cells produce TNF-α.
LCMV effects are mediated via TLR2/MyD88/Mal; CNS primary glial cells (passage 2 to 3) were challenged with LCMV and other TLR ligands for 18-24 hours in the presence of Brefeldin A at a concentration of 1 μg/ml. Cells were first stained with CD11b, which was used to distinguish microglial cells (CD11b positive subset) from astrocytes (CD11b negative subset). The intracellular expression of MCP-1 and TNF-α was determined by double staining with antibodies against mouse MCP-1 or TNF-α (Fig 2B). The expression of CD11b was used to distinguish microglial cells (CD11b+) from astrocytes (CD11b-). The percentage of each subset of cells expressing either MCP-1 (A) or TNF-α (B) was gated, analyzed and shown as bar graphs. Representatives of the FACS analysis were shown in the bottom panels of Fig 2a, 2b. LCMV-infected primary microglial cells were stained with antibodies against LCMV-NP, CD11b, and TNF-α. Cells were first gated on LCMV+CD11b+ and LCMV+CD11b- subsets of cells. The production of TNF-α by these two populations was separately gated (C). TLR3 and/or TLR4 KO primary mixed glial cells were included as TLR specificity controls. Values on the upper right quadrant represent the percentage of glial cells producing cytokines. One representative of at least five experiments is shown. (D) Confocal Microscopy: Cells were treated as described in the text, fixed with Paraformaldehyde, permeabilized and stained with anti-CD11b antibody (a marker for microglial cells) and anti-GFAP antibody (as a marker for astrocytes). Additionally, cells were incubated with anti-TNF-α antibodies for intracellular TNF production and Hoechst 33258 to stain nuclei.