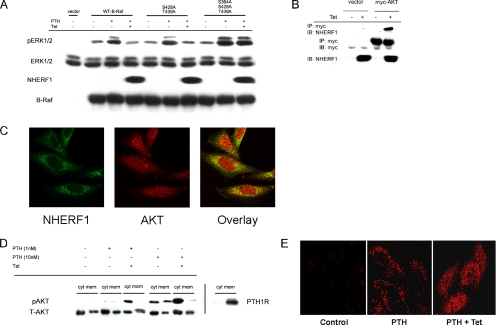
Figure 6.
NHERF1 effects on B-Raf regulatory domain. (A) Mutation of the inhibitory B-Raf regulatory domain AKT phosphorylation sites enhances PTH-stimulated ERK1/2 and prevents inhibition by NHERF1. CHO-N10-R3 cells were pretreated with 50 ng/ml Tet as indicated, and then they were transiently transfected with 1.0 μg DNA/well of empty vector, wild-type B-Raf, B-Raf(S428A,T439A), or B-Raf(S364A, S428A, T439A). After 48 h, the cells were serum starved for 3–5 h, and then they were pretreated with 20 μM H89 for 10 min before addition of 1 nM PTH for 10 min. Phosphorylated and total ERK1/2, NHERF1 and B-Raf in whole-cell lysates were determined by immunoblotting (IB). The results are representative of three independent experiments. (B) AKT directly interacts with NHERF1. CHO-N10-R3 cells were treated with 50 ng/ml Tet as indicated, and then they were transfected with myc-AKT. After 48 h, the interaction with NHERF1 was determined by coimmunoprecipitation (IP) and immunoblotting. (C) Colocalization of AKT and NHERF1. CHO-N10-R3 cells were treated with 50 ng/ml Tet for 48 h, and then they were fixed and incubated with polyclonal anti-AKT antibody and monoclonal anti-NHERF1 antibody. Fluorescent staining was analyzed by confocal microscopy as described in Materials and Methods. (D) NHERF1 blocks PTH-induced AKT translocation from membrane to cytosol. The extent of phospho-AKT in cytoplasm (cyt) and membrane (mem) was determined by immunoblotting of the respective fractions that were prepared as described in Materials and Methods. PTH1R was similarly analyzed. (E) Phospho-AKT localization. In control condition, pAKT is barely detectable. PTH (10 nM) for 10 min significantly increased pAKT in membrane punctae. Induction of NHERF1 restricts pAKT to cytoplasm upon PTH application.