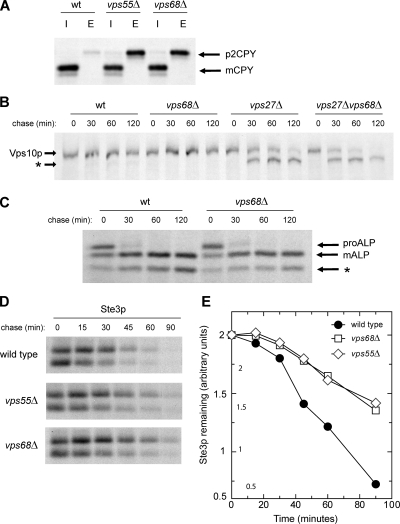
Figure 4.
Cargo sorting in vps68 and vps55 mutants. (A) Secretion of newly synthesized CPY. Strains were labeled with [35S]methionine for 10 min and chased for 60 min in the presence of 50 μg/ml unlabeled cysteine and methionine. CPY was immunoprecipitated from intracellular (I) and extracellular (E) fractions, analyzed by SDS-PAGE, and visualized by fluorography. Arrows indicate the position of Golgi-modified (p2) CPY and that of mature, vacuolar (m) CPY. (B) VPS68 acts downstream of the class E gene VPS27. Pulse-chase labeling and immunoprecipitation of Vps10p was carried out as described above using the following strains: wild-type (BLY1), vps27 (KEBY37), vps68 (MSY3), and vps27 vps68 (MSY11). Proteolytic cleavage of Vps10p in vps27 and vps27 vps68 double mutants generates a faster-migrating cleavage product (*). (C) Kinetics of ALP maturation are unchanged in vps68 mutants. ALP was immunoprecipitated from wild-type (RPY10) or vps68 cells (MSY2) that had been radiolabeled for 10 min with [35S]methionine and chased for the times indicated. PEP4-dependent cleavage converts precursor (pro) forms of ALP into the mature (m) form. Asterisk (*) indicates a commonly observed degradation product. (D and E) Degradation of Ste3p is slowed in vps68 mutants. Ste3p was immunoprecipitated from wild-type (RPY10), vps68 (MSY2), or vps55 (LCY434) cultures that had been radiolabeled with [35S]methionine for 10 min and chased with unlabeled cysteine and methionine for the times indicated. The total amount of Ste3 remaining at each time point was quantitated by densitometry. Representative images for wild-type, vps55, and vps68 strains are shown in D, and semilog plots are shown in E. Wild type, •; vps68, □; vps55, ◇.