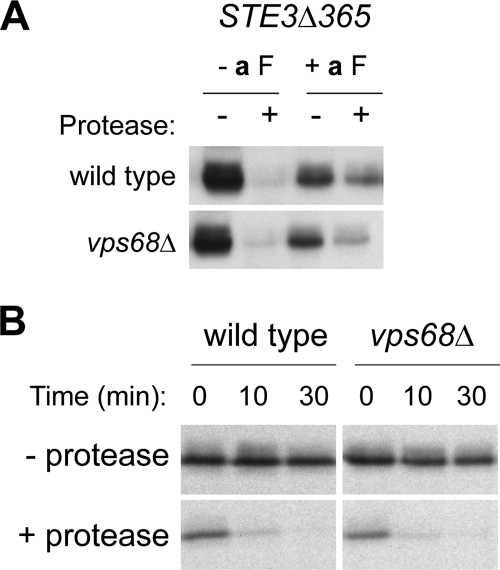
Figure 6.
Loss of VPS68 does not slow the endosome-to-cell surface recycling of Ste3Δ365p. (A) Ligand-dependent internalization of Ste3Δ365p. Wild type (NDY1181) and vps68 (BWY14) mutant cells were exposed to a-factor to induce endocytosis, and treated with protease to cleave surface-exposed Ste3Δ365 protein. Ste3Δ365p was detected in cell extracts by Western blotting with anti-HA mAb. (B) Cell surface recycling of internalized Ste3Δ365. Wild-type and vps68 strains expressing Ste3Δ365 were treated as described in A and incubated in fresh medium lacking a-factor. Aliquots removed at the times indicated were subjected to a second protease treatment. The time course with which the internal pool of Ste3Δ365 regains protease sensitivity provides a measure of cell surface recycling.