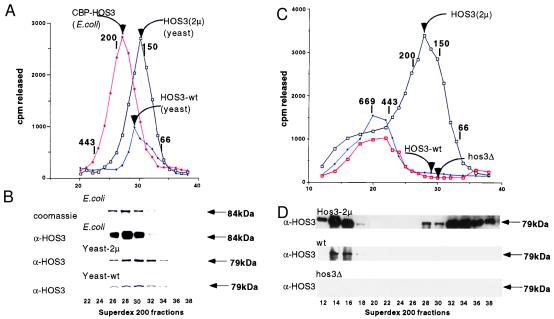
Figure 2.
HOS3 is present in a dimer-sized complex in both yeast and E. coli. (A) E. coli-expressed HOS3 activity containing the CBP-HOS3 chromatographs on Superdex-200 at an apparent molecular mass of 190 kDa. CBP-HOS3 was purified from E. coli by affinity chromatography on calmodulin-Sepharose as described in Experimental Procedures. Twenty-five microliters of each 0.5-ml fraction were used in a 1-hr incubation with 25,000 cpm total of [3H]acetyl-Hela histones. Activity ([3H]acetic acid released) is shown as a function of fraction number. HOS3 activity from yeast strains containing HOS3(2μ) or wild-type HOS3 was purified from nuclear extracts on DEAE-Sepharose, S-Sepharose, Mono S, and Superdex 200 as described in Experimental Procedures. Protein molecular mass standards are indicated above the chromatograph as 669 kDa (thyroglobulin), 443 kDa (apoferritin), 200 kDa (β-amylase), 150 kDa (alcohol dehydrogenase), and 66 kDa (bovine serum albumin). (B) Electrophoretic (SDS/PAGE) and immunological analysis of column fractions in A. Coomassie staining of E. coli CBP-HOS3 reveals the presence of an 84-kDa protein that comigrates with deacetylase activity. This protein reacts with anti-HOS3 antibody as detected by Western blotting (ECL, Amersham). Similar electrophoresis and Western analysis of the column fractions (A) of HOS3 deacetylase from HOS3(2μ) and HOS3 (wt) yeast indicates a 79-kDa protein that comigrates with the peak yeast activities. (C) Deacetylase activities from wild-type (YDS2), hos3Δ (SRYH3), or HOS3(2μ) (SRYH3gal) strains were partially purified on DEAE-Sepharose columns and then directly chromatographed by Superdex 200 gel filtration (1.0 × 46 cm column, 0.5-ml fractions). (D) Western blotting of Superdex 200 fractions from HOS3(2μ) (SRYH3gal), HOS3 wild type (YDS2), and hos3Δ (SRYH3) yeast.