Figure 4.
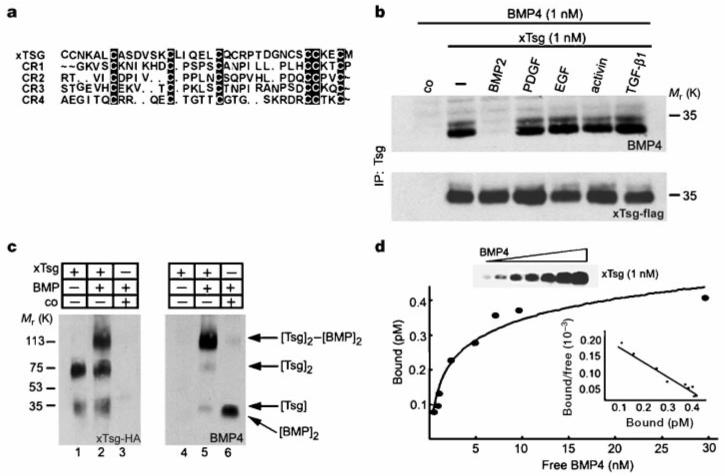
xTsg binds to BMP specifically and directly. a, Sequence similarities between Chordin cysteine-rich modules and the N-terminal domain of xTsg. b, Western blot analyses of BMP-4 (1.0 nM) bound to xTsg (1.0 nM) after immunoprecipitation by xTsg in the presence or absence of a 10-fold excess of various growth factors. The control (co) lanes in this and Figs 5-7 contain conditioned medium from mock-transfected 293T cells. To measure the recovery of xTsg after immunoprecipitation, the same membrane was stripped and probed for xTsg protein (bottom). c, Crosslinking analysis of xTsg-BMP-4 complexes with DSS. Left, immunoblot probed for xTsg. Right, the same membrane probed for BMP-4 after stripping. d, Equilibrium binding of increasing concentrations of BMP-4 (0.2-30 nM) to 1 nM Tsg. Binding was for 3 h at 4 °C, and bound and free BMP-4 were separated by immunoprecipitation; crosslinking agents were not used. The amount of bound BMP-4 was determined as described12. Each data point was in duplicate, and two independent experiments were performed for each protein. Scatchard analyses of the dTsg data gave an apparent Kd of 2.5 nM. The same results and Kd were obtained for the xTsg protein as illustrated by the anti-BMP-4 immunoblot (inset and data not shown).
