Figure 7.
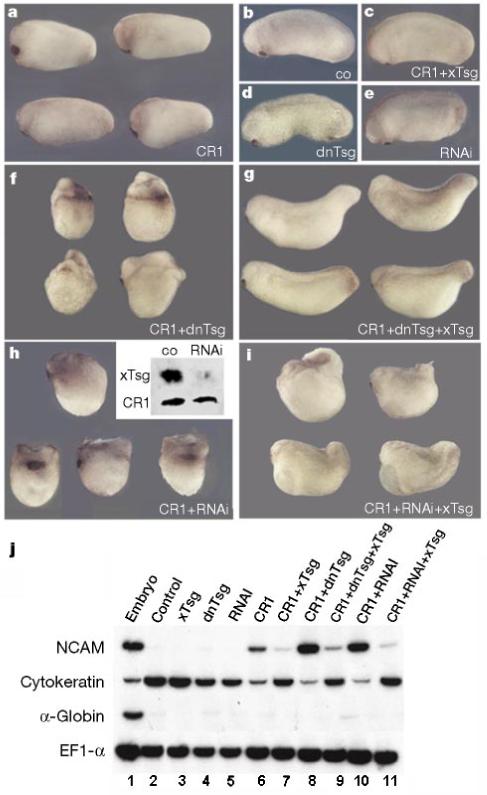
Loss of function of endogenous xTsg potentiates the activity of CR1 mRNA. Four-cell embryos were injected into each cell in the marginal region and cultured to stage 25. a, Injection of CR1 results in slight dorsalization compared with control uninjected embryos (b). c, Co-injection of xTsg and CR1 mRNA prevents dorsalization by CR1. Injection of dn-xTsg (d) or RNAi (e) disrupts the development of the perianal region leading to shortening of the tail at later stages (not shown); at these early tadpole stages the phenotype was minimal. Co-injection of CR1 and dn-xTsg mRNA (f) or RNAi (h) leads to strong synergistic dorsalization of the embryos, which can be partially rescued by co-injecting full-length xTsg mRNA (g, i). h, Inset, co-injection of HA-tagged xTsg mRNA and Myc-tagged CR1 mRNA with or without xTsg RNAi. Ectodermal explants were dissociated3, incubated for 14 h at 16 °C, and secreted proteins analysed by western blot. j, RT-PCR analysis of stage 25 ectodermal explants of embryos injected at the eight-cell stage. NCAM induction reflects inhibition of BMP signalling by CR1 (lane 6), which is potentiated by co-injection of dn-xTsg or RNAi (lanes 8 and 10). The induction of NCAM by CR1, as well as its potentiation by co-injection of dn-xTsg or RNAi, was inhibited by co-injection of wild-type xTsg (lanes 7, 9, 11). The epidermal marker gene cytokeratin is regulated reciprocally by the injected RNAs and serves as a reporter of endogenous BMP signals. α-Globin was used as a mesodermal marker and EF1-α as loading control. The amounts of mRNA per blastomere injected were CR1, 20 pg; xTsg, 200 pg; dn-xTsg, 100 pg; and RNAi, 100 pg.
