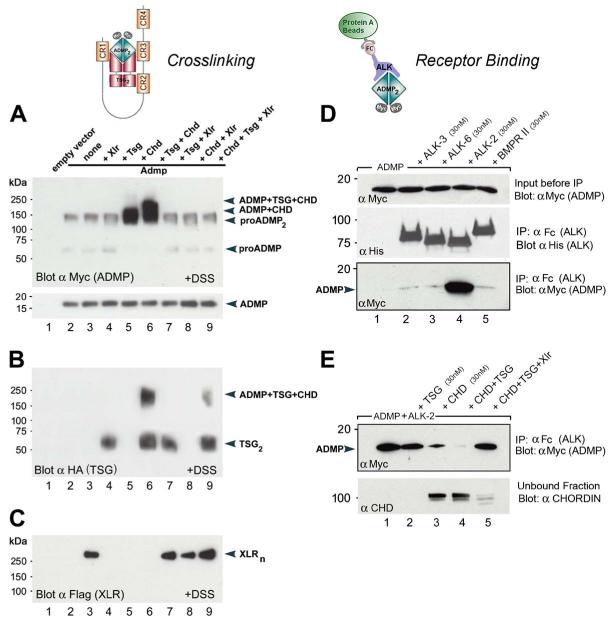
Figure 4. ADMP Binds to Chordin and Tsg, and Cleavage by Xolloid-Related Metalloproteinase Restores Binding to ALK-2 Receptor.
Supernatants of cocultures of 293T cells were used for crosslinking experiments (A-C). Pure recombinant proteins from R&D Systems were used for receptor binding assays (D and E).
(A) ADMP protein forms a binary complex with Chordin (lane 5) or a ternary complex with Chordin and Tsg (lane 6), which can be inactivated by Xolloid-related (lanes 8 and 9).
(B) Tsg protein forms a molecular complex with ADMP and Chordin proteins (lanes 6 and 9).
(C) Crosslinked Xlr enzyme runs as a discrete oligomer (Xlrn).
(D) ADMP binds specifically to the type I receptor ALK2 (lane 4) but not to BMPR-1a (ALK-3), BMPR-1b (ALK-6), or BMPR-II (lanes 2, 3, and 5). Other panels show loading controls.
(E) ADMP binding to ALK-2 is partially blocked by Chordin (top panel, lane 3) and completely inhibited by Chordin and Tsg (lane 4). Importantly, cleavage of Chordin by the metalloproteinase Xlr restores ADMP binding to ALK-2 (lane 5).