Figure 1. Detection of β-gal activity in vitro and in vivo.
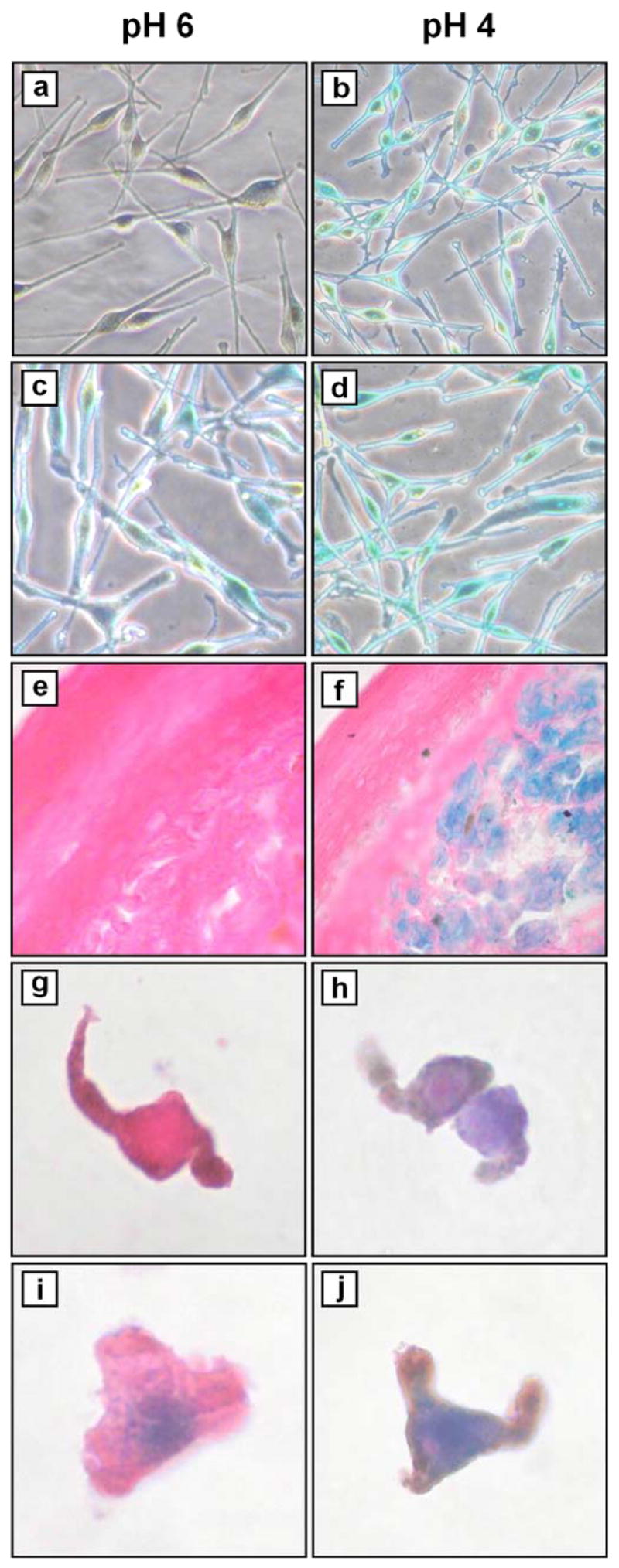
Melanocytes from (a, b) passage 2 (early) and (c, d) passage 12 (late) cultures were stained as described (Dimri et al., 1995). Briefly, cells were washed with PBS and fixed with 1% paraformaldehyde for 3 min at room temperature, then washed three times with PBS for 5 min each at room temperature. Staining was performed overnight in a non-CO2 enriched incubator at 37 °C using a solution (pH 4 or pH 6 as indicated) containing 40 mM sodium phosphate (dibasic), 40 mM citric acid, 150 mM NaCl, 2 mM MgCl2, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 1 mg/ml X-gal (5-bromo-4-chloro-3-indolyl-b-D-galactoside, Pierce Chemical Co., Rockford, IL). Cyanide salts and X-gal were added from freshly-made 100× stocks in PBS and dimethylformamide, respectively. Cells were then washed three times with PBS for 5 min each at room temperature, prior to microscopic examination and photography (×200). (e, f) nevus #5 (Table I). Frozen sections were fixed and stained as above (with additional staining at pH 5), followed by counterstaining with eosin, alcohol dehydration and mounting prior to microscopic examination and photography (×400). (g, h) Passage 3 melanocytes and (i, j) passage 15 melanocytes were trypsinized, washed with PBS, and resuspended in OCT prior to frozen sectioning. Sections were then fixed and stained in parallel with nevi, as above.
