Fig. 7. Kinetic pathways for fIX activation by fXIa.
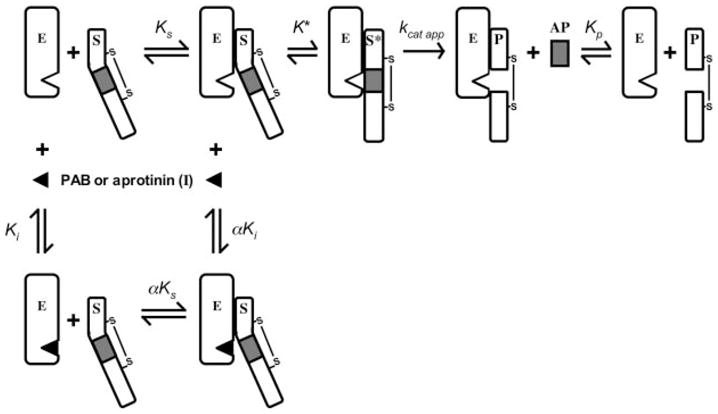
The model illustrates the conversion of fIX (S) to fIXaβ (P) by fXIa (E), with release of the activation peptide (AP) in the presence or absence of the reversible active site inhibitors pAB or aprotinin (I). The equilibrium dissociation constants for binding of S, I, and P to E are designated Ks, Ki, and Kp, respectively. The value for α is 2.7 ± 0.04 for inhibition with pAB and 6.3 ± 4.1 for aprotinin. Formation of the ES complex involves exosites on E remote from the active site. K* is the equilibrium constant for the docking interaction between S and E at the enzyme active site after binding at the exosite, S* is the substrate engaged at the enzyme active site, and kcat app represents catalysis. Note that kcat app is a term representing cleavage at both fIX activation sites and does not give specific information on cleavage at individual sites. The model shows one-half of the fXIa dimer and makes the assumption that each half-dimer activates fIX independently of the other half. An alternative model (not shown) could involve both catalytic domains of the dimer acting on one fIX molecule, with each active site cleaving one fIX activation site (49).
