Abstract
Objective
Recent literature has shown that common type atrial flutter (AFL) can recur late after cavotricuspid isthmus (CTI) catheter ablation using radiofrequency energy (RF). We report the long term outcome of a large group of patients undergoing CTI ablation using cryothermy for AFL in a single center.
Methods
Patients with AFL referred for CTI ablation were recruited prospectively from July 2001 to July 2006. Cryoablation was performed using a deflectable, 10.5 F, 6.5 mm tip catheter. CTI block was reassessed 30 min after the last application during isoproterenol infusion. Recurrences were evaluated by 12-lead ECG and 24 h Holter recording every clinic visit (1/3/6/9 and 12 months after the procedure and yearly thereafter) or if symptoms developed.
Results
The 180 enrolled patients had the following characteristics: 39 women (22%), mean age 58 years, no structural heart disease in 86 patients (48%), mean left atrium diameter 44 ± 7 mm and mean left ventricular ejection fraction 57 ± 7%. The average number of applications per patient was 7 (3 to 20) with a mean temperature and duration of −88°C and 3 min, respectively. Acute success was achieved in 95% (171) of the patients. There were no complications. After a mean follow-up of 27 ± 17 (from 12 to 60) months, the chronic success rate was 91%. The majority of the recurrences occurred within the first year post ablation. One hundred and twenty three patients had a history of atrial fibrillation (AF) prior to CTI ablation and 85 (69%) of those remained having AF after cryoablation. In 20 of 57 (35%) patients without a history of AF prior to CTI ablation, AF occurred during follow-up.
Conclusions
This prospective study showed a 91% chronic success rate (range 12 to 60 months) for cryoablation of the CTI in patients with common type AFL and ratified the frequent association of AF with AFL.
Keywords: Atrial flutter, Atrial fibrillation, Cryoablation, Long term follow-up, Cavotricuspid isthmus
Introduction
Ablation of the cavotricuspid isthmus (CTI) for the treatment of atrial flutter (AFL) has become standard practice. Most of the procedures are performed using radiofrequency energy (RF) [1–5]. High chronic success rates are described but the majority of the data comes from a relatively short term follow-up [1–13]. A recent study by Chinitz et al. [14] reported some interesting data regarding the long term follow-up of 80 patients with common type AFL who underwent CTI ablation using RF. They found a 12.5% (ten patients) recurrence rate at an average of 21 months after the procedure with most patients having a recurrence after the first year post ablation. Our prior experience using cryothermy in a limited number of patients [15] also showed that AFL may recur 1 year after CTI cryoablation.
The purpose of this study was to evaluate the long term outcome of CTI cryoablation in a large patient population with common type AFL in a single center.
Methods
One hundred and eighty patients with sustained symptomatic common type AFL referred for CTI ablation were enrolled prospectively from July 2001 to July 2006 in our institution. Signed written consent, approved by the local ethics committee, was obtained from all participants.
Before CTI cryoablation, anticoagulation with warfarin aiming a therapeutic international normalized ratio of 2 to 3 was kept for at least 3 weeks. Antiarrhythmic drugs were not discontinued before ablation.
Electrophysiologic study and ablation
We focused our study on the clinical aspects and long term follow-up of patients with AFL who were submitted to CTI cryoablation.
Our CTI cryoablation protocol has been already reported [15]. Briefly, our methodology was as follows: under local anesthesia and via the femoral route, a decapolar catheter is positioned in the distal coronary sinus (for evaluation of left atrial activation), a duodecapolar catheter (2-mm interelectrode spacing, Halo catheter, Biosense Webster, Baldwin Park, CA) for mapping the right atrial lateral wall and a quadripolar catheter in the His bundle area. A deflectable, 10.5 F, 6.5 mm tip cryoablation catheter (CryoCor Inc., San Diego, CA) is inserted into the right atrium through a 12F, 65-cm-long sheath (DAIG, St. Jude Medical Inc., St. Paul, MN) [15–19].
Entrainment to confirm the isthmus dependence of the AFL circuit was performed in every patient in whom AFL was present or could be induced at the start of the procedure, according to previously published techniques [2, 10, 15, 16, 20, 21]. If AF, requiring cardioversion, was present or developed during stimulation or if we were unable, even under isoproterenol infusion, to induce AFL, CTI ablation was performed during sinus rhythm. Linear lesions were created by use of a point-by-point technique with gradual pullback of the cryocatheter in a ventricular atrial fashion. The first application was delivered at the ventricular insertion of the isthmus and applications were continued for an average of 3 min. In patients in whom ablation of the posterior isthmus proved insufficient, an attempt was made to ablate the septal isthmus (four patients). After documentation of bidirectional isthmus conduction block, the atrial pacing (from the proximal coronary sinus) protocol (up to three atrial extrastimuli at three pacing cycle lengths and incremental atrial pacing) was performed without and with isoproterenol infusion (1 to 3 μg/min). Acute success was defined as bidirectional isthmus conduction block, 30 min after the last application without and with isoproterenol infusion [22].
All patients were studied in the fasting state without sedation. Those presenting in AF while on the catheterization table were converted to sinus rhythm by internal or external cardioversion. During the procedure intravenous heparin was given as a 100-IU/kg bolus dose after the venous sheaths were inserted. The 12-lead ECG and intracardiac electrograms were recorded and stored by the BARD Labsystem PRO.
Post ablation management
All patients were monitored in hospital for 24 h and oral anticoagulation was started the day of ablation. Antiarrhythmic drugs (AAD) were stopped after the procedure in patients without a history of AF; in those with AF/AFL the same AAD were continued. All patients had anticoagulation for at least 1 month. Subsequently, the need for chronic anticoagulation was assessed by the amount of recurrences of AFL/AF and the presence of risk factors for thromboembolic events.
All patients had a 12-lead ECG and a 24 h Holter recording at discharge and during each clinic visit (1 month, 3, 6, 9, 12 months and yearly thereafter) or earlier if they had symptoms.
Due to the logistics of the Maastricht area—and also the presence of a dedicated research nurse (S. P.) who was available to address patients’ concerns and questions at any time—we were able to follow every patient on an individual basis.
Statistical analysis
Continuous variables are presented as mean ± SD, where appropriate. In cases of a non-Gaussian distribution, medians and quartiles are given. Categorical variables are expressed as numbers and percentages of patients.
Statistical analysis was performed using the Student t test for unpaired data. All values were considered significant at P < 0.05.
The authors had full access to the data and take responsibility for its integrity.
Results
Of the 180 enrolled patients, 39 patients (22%) were women with a mean age of 58 (from 18 to 80) years. More than half of the patients (92 patients, 52%) had structural heart disease: arterial hypertension: 57 patients, coronary artery disease: 22 patients, valvular heart disease: 13 patients, congenital heart disease: 11 patients, idiopathic cardiomyopathy: 18 patients. Counterclockwise AFL was documented in 91% (164) of the patients and clockwise AFL in 9% (16 patients).
The mean left atrial diameter and the mean left ventricular ejection fraction were 44 ± 7 mm and 57 ± 7%, respectively. A prior history of AF was present in 123 (69%) of the patients. The clinical characteristics of the patients, related to the presence or absence of AF before ablation, are described in Table 1. Note that AF during follow-up is significantly higher in the group with a prior history of this arrhythmia.
Table 1.
Characteristics of the 180 patients with atrial flutter referred for CTI cryoablation related to the presence or absence of atrial fibrillation AF before ablation
| AF/AFL patients (123 patients), 69% | AFL only (57 patients), 31% | p value | |
|---|---|---|---|
| Age (year) | 57 ± 13 | 58 ± 13 | ns |
| Women | 19% (23 patients) | 28% (16 patients) | ns |
| No SHD | 55% (68 patients) | 32% (18 patients) | < 0.05 |
| LAd (cm) | 4.4 | 4.5 | ns |
| LVEF (%) | 58 | 55 | ns |
| Acute failuresa | 5% (6 patients) | 5% (3 patients) | ns |
| AF in long term follow up | 69% (85 patients) | 35% (20 patients) | < 0.05 |
aPatients in whom CTI cryoablation did not result in bidirectional block (failed procedure).
AF Atrial fibrillation, AFL atrial flutter, CTI cavotricuspid isthmus, Lad left atrium diameter, LVEF left ventricular ejection fraction, SHD structural heart disease
Total fluoroscopic—mean of 30 ± 18 min (range, 12 to 152 min)—and procedure times—mean of 2.6 ± 1.1 h (range, 1 to 6.5 h)—decreased throughout our study with a long duration of a procedure and fluoroscopy being attributed mainly to the learning curve of a new technology. An average of 7 (3 to 20) applications per patient were delivered with a mean temperature and duration of −88°C and 3 min, respectively.
The acute success rate for cryoablation of the CTI was 95% (171 patients). There were no complications. Of the nine patients in whom bidirectional CTI block was not achieved, three underwent a successful reablation. The other six patients had much improvement of their symptoms (despite an incomplete line) and preferred not to have another procedure.
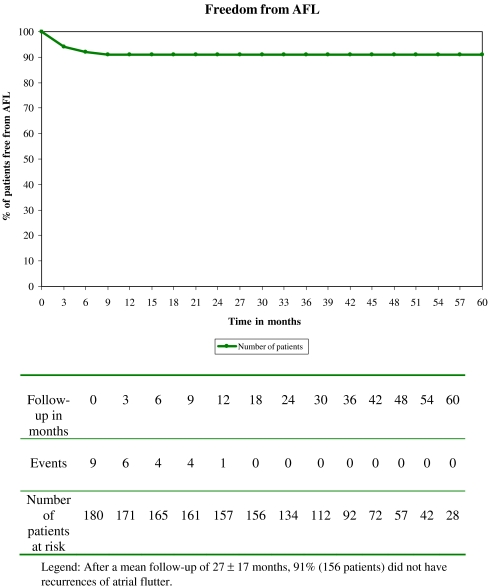
After a mean follow-up of 27 ± 17 (range from 12 to 60) months, recurrences of symptomatic AFL occurred in 15 patients (9%) resulting in a 91% chronic success rate. Those recurrences occurred in six patients within the first 3 months post ablation, in four patients from 3 to 6 months post ablation, in four patients from 6 to 9 months post ablation and in one patient at 14 months post ablation (Fig. 1). Ten of those 15 patients underwent a second successful cryoablation of the CTI. The other five had not only reablation of the isthmus but also pulmonary vein cryoisolation (PVI) for AF during the second procedure.
Fig. 1.
Percentage of patients (171 successfully ablated) free of common type atrial flutter over time
Despite the success as far as AFL was concerned, AF was still present in 85 patients (69%) with a prior history of this arrhythmia. Those patients were treated by AAD (69 patients, 81%), PVI (14 patients, 16%) or AV nodal ablation with pacemaker implantation (two patients, 3%). New episodes of AF developed in 20 (35%) of those 57 patients without documented AF prior to CTI cryoablation and were all controlled by AAD.
Discussion
Main findings
Our current study showed a 91% chronic success rate of CTI cryoablation in a large population (180 patients) with AFL followed for a long period of time (1 to 5 years, mean of 27 months).
According to the most recent ACC/AHA/ESC guidelines for the management of supraventricular arrhythmias CTI ablation is the only therapy with a class I indication for the long term management of AFL [2]. The majority of those ablations are done using RF energy. Concerning the follow-up length of those procedures, most of the literature available reports on a relatively short period, the majority of them being less than 2 years. However Chinitz et al. [14] published a study of 80 patients with AFL submitted to CTI ablation using RF that had up to 6 years follow-up. Interestingly, they found a 12.5% recurrence of AFL that occurred on an average of 21 months post ablation, ratifying the need of data with a longer follow-up. It is important to keep in mind though, that arrhythmias after ablation do not always correlate with symptoms [23, 24] and one could question if those patients with such a late recurrence had those episodes much earlier than what is reported. That might be one reason why our results, where most of our recurrences happened within 1 year, are discrepant with those from their study. The intrinsic characteristics of our hospital, our clinical follow-up and the population of Maastricht could be responsible for our ability to find those recurrences earlier.
Regarding cryothermy as energy source, a 9 months follow-up study in 39 patients undergoing CTI cryoablation with a different system was reported [25]. They achieved a chronic success rate of 100% in the cryoablated group, despite documenting reconduction through the isthmus in 31% of patients during a 3 month follow-up electrophysiologic study. The CRAAFT trial presented the results in 32 patients with AFL submitted to either cryo- or RF ablation of the CTI [26]. They report an 84% success rate after a follow-up of 14 months. Those two small studies, with a relatively short follow-up, reported similar success rates with RF and cryothermy for AFL ablation. The results from our study—which included a significant larger patient population (180) with a longer follow-up (1 to 5 years, mean of 27 months)—reinforces the effectiveness of cryothermy for the treatment of AFL, being the outcomes comparable to those reported in the literature using RF (where most outcome data also comes from non invasive follow-up) [1, 4–7, 11, 12, 27, 28]. Therefore, cryothermy can be considered as an efficient energy source for the treatment of common type AFL.
The relation of AF with AFL
The close relation between AF and AFL is well described [1, 5–14, 21, 29–39]. Our data showed a high prevalence of AF (123 out of 180 patients, 69%) in patients with predominant AFL referred for CTI ablation. A recent study by Ellis and colleagues [40] strengthened even more this association. They followed 363 patients with lone AFL who underwent CTI RF ablation—during a mean follow-up of 39 months—and reported an 82% incidence of drug refractory AF in their patient population.
The new development of AF in our patient population without a prior history of AF preceding AFL ablation may be a sign of an already present electrical and morphological change in the right and left atria. If in addition, a functional or anatomical line of block between the two venae cavae (or elsewhere) occurs, atypical AFL(s) may develop either in the right (because the CTI is already ablated) or in the left atrium [11, 14, 21, 31, 32, 37–39].
Study limitations
Our recurrence data rely mostly on the subjective assessment by the patient, like the great majority of RF data [1, 4, 6–8, 12]. Only an objective measurement (such as a repeat electrophysiological study with documented bidirectional block) will determine the long lasting effect of CTI ablation for the treatment of common type AFL.
Conclusions
In this prospectively studied large population of patients with common type AFL, cryoablation of the CTI has a 91% chronic success rate during long term follow-up (range 1 to 5 years, mean of 27 months). These results are similar to those obtained with RF, validating cryothermy as an efficient alternative energy source. We also were able to ratify the frequent association of AF with AFL.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Disclosures: Drs Rodriguez and Timmermans have received a modest research grant from CryoCor, San Diego, CA, USA. Dr Wellens is a consultant for CryoCor, San Diego, CA, USA.
References
- 1.Anne, W., Willems, R., Adriaenssens, B., Adams, J., Ector, H., & Heidbuchel, H. (2006). Long-term symptomatic benefit after radiofrequency catheter ablation for atrial flutter despite a high incidence of post-procedural atrial fibrillation. Acta Cardiologica, 61, 75–82. [DOI] [PubMed]
- 2.Blomstrom-Lundqvist, C., Scheinman, M. M., Aliot, E. M., Alpert, J. S., Calkins, H., Camm, A. J., et al. (2003). ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias—executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Circulation, 108, 1871–1909. [DOI] [PubMed]
- 3.Marrouche, N. F., Schweikert, R., Saliba, W., Pavia, S. V., Martin, D. O., Dresing, T., et al. (2003). Use of different catheter ablation technologies for treatment of typical atrial flutter: Acute results and long-term follow-up. Pacing and Clinical Electrophysiology, 26, 743–746. [DOI] [PubMed]
- 4.Natale, A., Newby, K. H., Pisano, E., Leonelli, F., Fanelli, R., Potenza, D., et al. (2000). Prospective randomized comparison of antiarrhythmic therapy versus first-line radiofrequency ablation in patients with atrial flutter. Journal of the American College of Cardiology, 35, 1898–1904. [DOI] [PubMed]
- 5.Schmieder, S., Ndrepepa, G., Dong, J., Zrenner, B., Schreieck, J., Schneider, M. A., et al. (2003). Acute and long-term results of radiofrequency ablation of common atrial flutter and the influence of the right atrial isthmus ablation on the occurrence of atrial fibrillation. European Heart Journal, 24, 956–962. [DOI] [PubMed]
- 6.Bertaglia, E., Zoppo, F., Bonso, A., Proclemer, A., Verlato, R., Coro, L., et al. (2004). Long term follow up of radiofrequency catheter ablation of atrial flutter: clinical course and predictors of atrial fibrillation occurrence. Heart, 90, 59–63. [DOI] [PMC free article] [PubMed]
- 7.Bertaglia, E., Bonso, A., Zoppo, F., Proclemer, A., Verlato, R., Coro, L., et al. (2004). Different clinical courses and predictors of atrial fibrillation occurrence after transisthmic ablation in patients with preablation lone atrial flutter, coexistent atrial fibrillation, and drug induced atrial flutter. Pacing and Clinical Electrophysiology, 27, 1507–1512. [DOI] [PubMed]
- 8.Bottoni, N., Donateo, P., Quartieri, F., Tomasi, C., Oddone, D., Lolli, G., et al. (2004). Outcome after cavo-tricuspid isthmus ablation in patients with recurrent atrial fibrillation and drug-related typical atrial flutter. American Journal of Cardiology, 94, 504–508. [DOI] [PubMed]
- 9.Da, C. A., Romeyer, C., Mourot, S., Messier, M., Cerisier, A., Faure, E., et al. (2002). Factors associated with early atrial fibrillation after ablation of common atrial flutter. A single centre prospective study. European Heart Journal, 23, 498–506. [DOI] [PubMed]
- 10.Da, C. A., Romeyer-Bouchard, C., Zarqane-Sliman, N., Messier, M., Samuel, B., Kihel, A., et al. (2005). Impact of first line radiofrequency ablation in patients with lone atrial flutter on the long term risk of subsequent atrial fibrillation. Heart, 91, 97–98. [DOI] [PMC free article] [PubMed]
- 11.Delise, P., Sitta, N., Coro, L., Marras, E., Sciarra, L., Bocchino, M., et al. (2006). Common atrial flutter and atrial fibrillation are not always two stages of the same disease. A long-term follow-up study in patients with atrial flutter treated with cavo-tricuspid isthmus ablation. Journal of Cardiovascular Medicine (Hagerstown), 7, 800–805. [DOI] [PubMed]
- 12.Hsieh, M. H., Tai, C. T., Chiang, C. E., Tsai, C. F., Yu, W. C., Chen, Y. J., et al. (2002). Recurrent atrial flutter and atrial fibrillation after catheter ablation of the cavotricuspid isthmus: a very long-term follow-up of 333 patients. Journal of Interventional Cardiac Electrophysiology, 7, 225–231. [DOI] [PubMed]
- 13.Husser, D., Bollmann, A., Kang, S., Girsky, M. J., Lerman, R. D., Cannom, D. S., et al. (2004). Effectiveness of catheter ablation for coexisting atrial fibrillation and atrial flutter. American Journal of Cardiology, 94, 666–668. [DOI] [PubMed]
- 14.Chinitz, J. S., Gerstenfeld, E. P., Marchlinski, F. E., & Callans, D. J. (2007). Atrial fibrillation is common after ablation of isolated atrial flutter during long-term follow-up. Heart Rhythm, 4, 1029–1033. [DOI] [PubMed]
- 15.Manusama, R., Timmermans, C., Limon, F., Philippens, S., Crijns, H. J., & Rodriguez, L. M. (2004). Catheter-based cryoablation permanently cures patients with common atrial flutter. Circulation, 109, 1636–1639. [DOI] [PubMed]
- 16.Manusama, R., Timmermans, C., Philippens, S., Crijns, H. J., Ayers, G. M., & Rodriguez, L. M. (2004). Single cryothermia applications of less than five minutes produce permanent cavotricuspid isthmus block in humans. Heart Rhythm, 1, 594–599. [DOI] [PubMed]
- 17.Rodriguez, L. M., Geller, J. C., Tse, H. F., Timmermans, C., Reek, S., Lee, K. L., et al. (2002). Acute results of transvenous cryoablation of supraventricular tachycardia (atrial fibrillation, atrial flutter, Wolff–Parkinson–White syndrome, atrioventricular nodal reentry tachycardia). Journal of Cardiovascular Electrophysiology, 13, 1082–1089. [DOI] [PubMed]
- 18.Rodriguez, L. M., & Timmermans, C. (2004). Transvenous cryoablation of cardiac arrhythmias. Technology Cancer Research Treatment, 3, 515–524. [DOI] [PubMed]
- 19.Timmermans, C., Ayers, G. M., Crijns, H. J., & Rodriguez, L. M. (2003). Randomized study comparing radiofrequency ablation with cryoablation for the treatment of atrial flutter with emphasis on pain perception. Circulation, 107, 1250–1252. [DOI] [PubMed]
- 20.Andrew, P., & Montenero, A. S. (2007). Atrial flutter: A focus on treatment options for a common supraventricular tachyarrhythmia. Journal of Cardiovascular Medicine (Hagerstown), 8, 558–567. [DOI] [PubMed]
- 21.Camm, A. J., & Obel, O. A. (1996). Epidemiology and mechanism of atrial fibrillation and atrial flutter. American Journal of Cardiology, 78, 3–11. [DOI] [PubMed]
- 22.Nabar, A., Rodriguez, L. M., Timmermans, C., Smeets, J. L., & Wellens, H. J. (1999). Isoproterenol to evaluate resumption of conduction after right atrial isthmus ablation in type I atrial flutter. Circulation, 99, 3286–3291. [DOI] [PubMed]
- 23.Klemm, H. U., Ventura, R., Rostock, T., Brandstrup, B., Risius, T., Meinertz, T., et al. (2006). Correlation of symptoms to ECG diagnosis following atrial fibrillation ablation. Journal of Cardiovascular Electrophysiology, 17, 146–150. [DOI] [PubMed]
- 24.Hindricks, G., Piorkowski, C., Tanner, H., Kobza, R., Gerds-Li, J. H., Carbucicchio, C., et al. (2005). Perception of atrial fibrillation before and after radiofrequency catheter ablation: Relevance of asymptomatic arrhythmia recurrence. Circulation, 112, 307–313. [DOI] [PubMed]
- 25.Montenero, A. S., Bruno, N., Antonelli, A., Mangiameli, D., Barbieri, L., Andrew, P., et al. (2005). Long-term efficacy of cryo catheter ablation for the treatment of atrial flutter: Results from a repeat electrophysiologic study. Journal of the American College of Cardiology, 45, 573–580. [DOI] [PubMed]
- 26.Collins, N. J., Barlow, M., Varghese, P., & Leitch, J. (2006). Cryoablation versus Radiofrequency Ablation in the treatment of Atrial Flutter trial (CRAAFT). Journal of Interventional Cardiac Electrophysiology, 16, 1–5. [DOI] [PubMed]
- 27.Nabar, A., Rodriguez, L. M., Timmermans, C., Smeets, J. L., & Wellens, H. J. (1999). Radiofrequency ablation of “class IC atrial flutter” in patients with resistant atrial fibrillation. American Journal of Cardiology, 83, 785–787, A10. [DOI] [PubMed]
- 28.Nabar, A., Rodriguez, L. M., Timmermans, C., van den, D. A., Smeets, J. L., & Wellens, H. J. (1999). Effect of right atrial isthmus ablation on the occurrence of atrial fibrillation: observations in four patient groups having type I atrial flutter with or without associated atrial fibrillation. Circulation, 99, 1441–1445. [DOI] [PubMed]
- 29.Calo, L., Lamberti, F., Loricchio, M. L., De, R. E., Bianconi, L., Pandozi, C., et al. (2005). Atrial flutter and atrial fibrillation: Which relationship? New insights into the electrophysiological mechanisms and catheter ablation treatment. Italian Heart Journal, 6, 368–373. [PubMed]
- 30.Chugh, A., Latchamsetty, R., Oral, H., Elmouchi, D., Tschopp, D., Reich, S., Igic, P., et al. (2006). Characteristics of cavotricuspid isthmus-dependent atrial flutter after left atrial ablation of atrial fibrillation. Circulation, 113, 609–615. [DOI] [PubMed]
- 31.Fischer, A., & Mehta, D. (2002). Atrial fibrillation after atrial flutter ablation. Journal of Interventional Cardiac Electrophysiology, 6, 181–182. [DOI] [PubMed]
- 32.Horvath, G., Goldberger, J. J., & Kadish, A. H. (2000). Simultaneous occurrence of atrial fibrillation and atrial flutter. Journal of Cardiovascular Electrophysiology, 11, 849–858. [DOI] [PubMed]
- 33.Hsieh, M. H., Tai, C. T., Tsai, C. F., Yu, W. C., Lin, W. S., Huang, J. L., et al. (2001). Mechanism of spontaneous transition from typical atrial flutter to atrial fibrillation: Role of ectopic atrial fibrillation foci. Pacing and Clinical Electrophysiology, 24, 46–52. [DOI] [PubMed]
- 34.Hsieh, M. H., Tai, C. T., Chan, P., & Chen, S. A. (2004). Spontaneous transition from atrial fibrillation to typical atrial flutter during catheter ablation of the pulmonary vein. Journal of Interventional Cardiac Electrophysiology, 10, 289–291. [DOI] [PubMed]
- 35.Kumagai, K., Tojo, H., Yasuda, T., Noguchi, H., Matsumoto, N., Nakashima, H., et al. (2000). Treatment of mixed atrial fibrillation and typical atrial flutter by hybrid catheter ablation. Pacing and Clinical Electrophysiology, 23, 1839–1842. [DOI] [PubMed]
- 36.Lelorier, P., Humphries, K. H., Krahn, A., Connolly, S. J., Talajic, M., Green, M., et al. (2004). Prognostic differences between atrial fibrillation and atrial flutter. American Journal of Cardiology, 93, 647–649. [DOI] [PubMed]
- 37.Morton, J. B., Byrne, M. J., Power, J. M., Raman, J., & Kalman, J. M. (2002). Electrical remodeling of the atrium in an anatomic model of atrial flutter: Relationship between substrate and triggers for conversion to atrial fibrillation. Circulation, 105, 258–264. [DOI] [PubMed]
- 38.Ramanna, H., De Bakker, J. M., & Hauer, R. N. (2005). Mechanism of propensity to atrial fibrillation in patients undergoing isthmus ablation for typical atrial flutter. Journal of Cardiovascular Electrophysiology, 16, 167–172. [DOI] [PubMed]
- 39.Waldo, A. L. (2005). The interrelationship between atrial fibrillation and atrial flutter. Progress in Cardiovascular Diseases, 48, 41–56. [DOI] [PubMed]
- 40.Ellis, K., Wazni, O., Marrouche, N., Martin, D., Gillinov, M., McCarthy, P., et al. (2007). Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. Journal of Cardiovascular Electrophysiology, 18, 799–802. [DOI] [PubMed]