FIGURE 5. Relationship between glutathione redox potential, GSH/GSSG ratio, and GSH pool size.
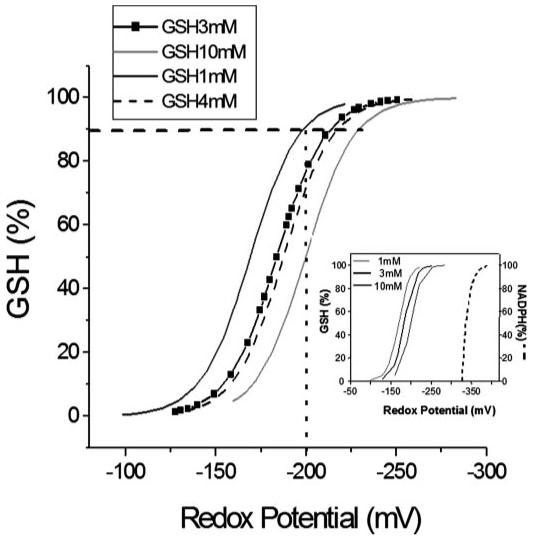
The glutathione redox potential, Ehc, was calculated according to Equation 1 in the text. The main panel shows how the total concentration of GSH influences the Ehc midpoint potential and how the pool size influences the % oxidation of the GSH pool. For example, if 10% of the GSH is oxidized to GSSG (horizontal dashed line), then for an initial GSH concentration of 10 mm, Ehc = -230 mV; for 4 mm, Ehc = -218 mV; for 3 mm, Ehc = -214 mV; and for 1 mm, Ehc = -200 mV. Thus, a cell with 10 mm GSH will have a higher reduction potential and a higher reducing capacity than one containing 1 mm GSH. The inset shows the reduction potential of NADP+/NADPH, whose negative redox potential (-400 mV (6)) makes it a key electron donor for the GSH system, for other redox systems (1), and for biosynthetic reactions. In general, NADPH is a cofactor in reductive (biosynthetic) reactions and serves as a source of electrons, whereas NAD+-dependent reactions are oxidative (catabolic) reactions where NAD+ serves as a sink for electrons. As opposed to the NADH/NAD+ ratio (= 100:1), the NADPH/NADP+ ratio is much lower (1:100) in cells and tissues (for review see Refs. 1, 6). The numbers at the top left of the inset correspond to GSH concentrations whose redox potential is represented in the plot (three continuous lines), and NADPH redox potential is shown in dashed line.
