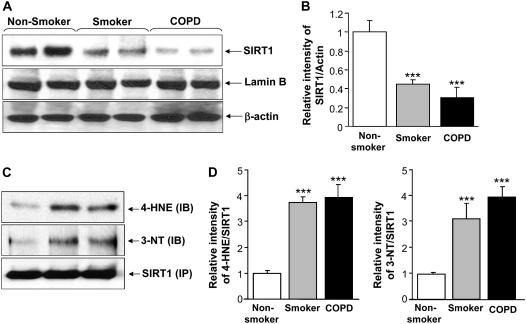
Figure 1.
Decreased levels of sirtuin (SIRT1) protein in lung tissue of smokers and patients with chronic obstructive pulmonary disease (COPD). (A) Western blot analysis of SIRT1 in soluble nuclear proteins (30 μg) extracted from the lung tissue of nonsmokers (n = 10), smokers (n = 10), and patients with COPD (n = 17). The proteins were electrophoresed on a 7.5% polyacrylamide gel electrophoresis and electroblotted onto a nitrocellulose membrane. The level of SIRT1 protein was determined using mouse monoclonal anti-SIRT1 antibody. The purity of nuclear extract was shown by the presence of lamin B (nuclear envelope protein) and the absence of the cytoskeletal protein α-tubulin (not shown). (B) After densitometric analysis, the values were normalized against the loading control, β-actin. The relative level (% of control) of SIRT1 showed decreased levels of nuclear SIRT1 protein in the lung tissues of smokers and patients with COPD. (C) SIRT1 protein was immunoprecipitated from the nuclear extract of lung homogenates. The levels of SIRT1 adducts with 4-hydroxy-2-nonenol (4-HNE) and nitration of tyrosine residues on SIRT1 were analyzed by immunoblotting with anti–4-HNE and anti–3-nitrotyrosine (3-NT) antibodies, respectively. Equal amount of immunoprecipitated SIRT1 protein (100 μg) was used for Western blotting. (D) Relative intensity of 4-HNE/SIRT1 and 3-NT/SIRT1 represents the increased post-translational modifications of SIRT1 protein in lungs of smokers and patients with COPD compared with nonsmokers. A representative blot is shown, which was obtained from several blotting experiments. Results are expressed as mean ± SEM. ***P < 0.001, significant compared with nonsmokers.