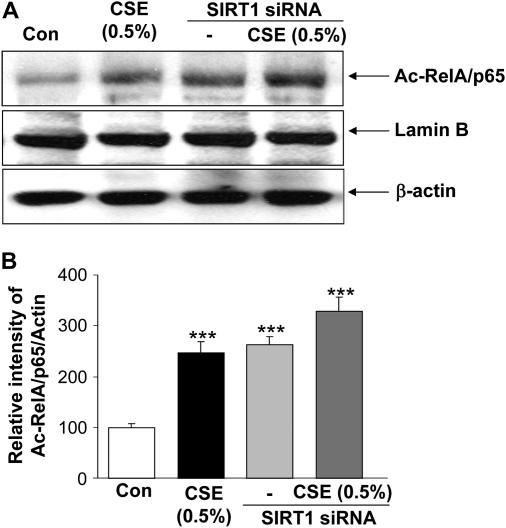
Figure 10.
siRNA silencing of sirtuin (SIRT1) augmented the cigarette smoke extract (CSE)–mediated acetylation of RelA/p65 nuclear factor (NF)-κB. Monocyte–macrophage (MonoMac6) cells were transfected with predesigned human SIRT1 siRNA duplex (100 nM) using DharmaFect2 transfection reagent for 36–48 hours and then treated with CSE (0.5%) for 12 hours. Nontargeting scrambled siRNA was used as a negative control. Actin was measured as a loading control. (A) Acetylation of RelA/p65 NF-κB was determined using rabbit anti-Ac-RelA/p65 (K310) antibody in the soluble nuclear extract. The purity of nuclear extract was shown by the presence of lamin B (nuclear envelope protein) and the absence of the cytoskeletal protein α-tubulin (bands not shown). (B) The relative level (% of control) of Ac-RelA/p65 showed increased acetylation of nuclear RelA/p65 in response to SIRT1 knockdown and/or CSE treatment. Each value is the mean ± SEM of triplicate determinations (n = 3). ***P < 0.001, significant compared with control.