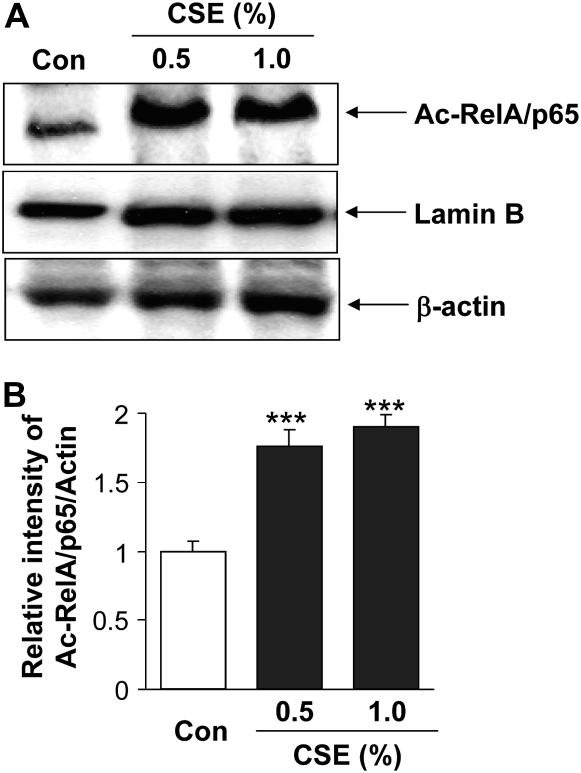
Figure 9.
Cigarette smoke extract (CSE)–mediated decrease in sirtuin (SIRT1) level was associated with increased acetylation of RelA/p65 nuclear factor (NF)-κB. (A) Monocyte–macrophage (MonoMac6) cells were treated with CSE (0.5 and 1.0%) for 4 hours. Acetylation of the lysine residue (K310) on RelA/p65 NF-κB protein was determined in soluble nuclear proteins (30 μg) by Western blotting using anti–acetyl RelA/p65 (K310) antibody. β-Actin was measured as a loading control. Lamin B (nuclear envelope protein) and the absence of the cytoskeletal protein α-tubulin (bands not shown) were measured to confirm the purity of nuclear extracts. (B) The relative density (% of control) of acetylated RelA/p65 NF-κB in nuclear fraction of MonoMac6 cells showed increased acetylation of RelA/p65 NF-κB in response to CSE treatment at 4 hours. Each histogram represents the means ± SEM (n = 3). ***P < 0.001, compared with control values.