Figure 2. Reduced Pax3 levels result in disorganization of Pax3neo/neo tongue musculature.
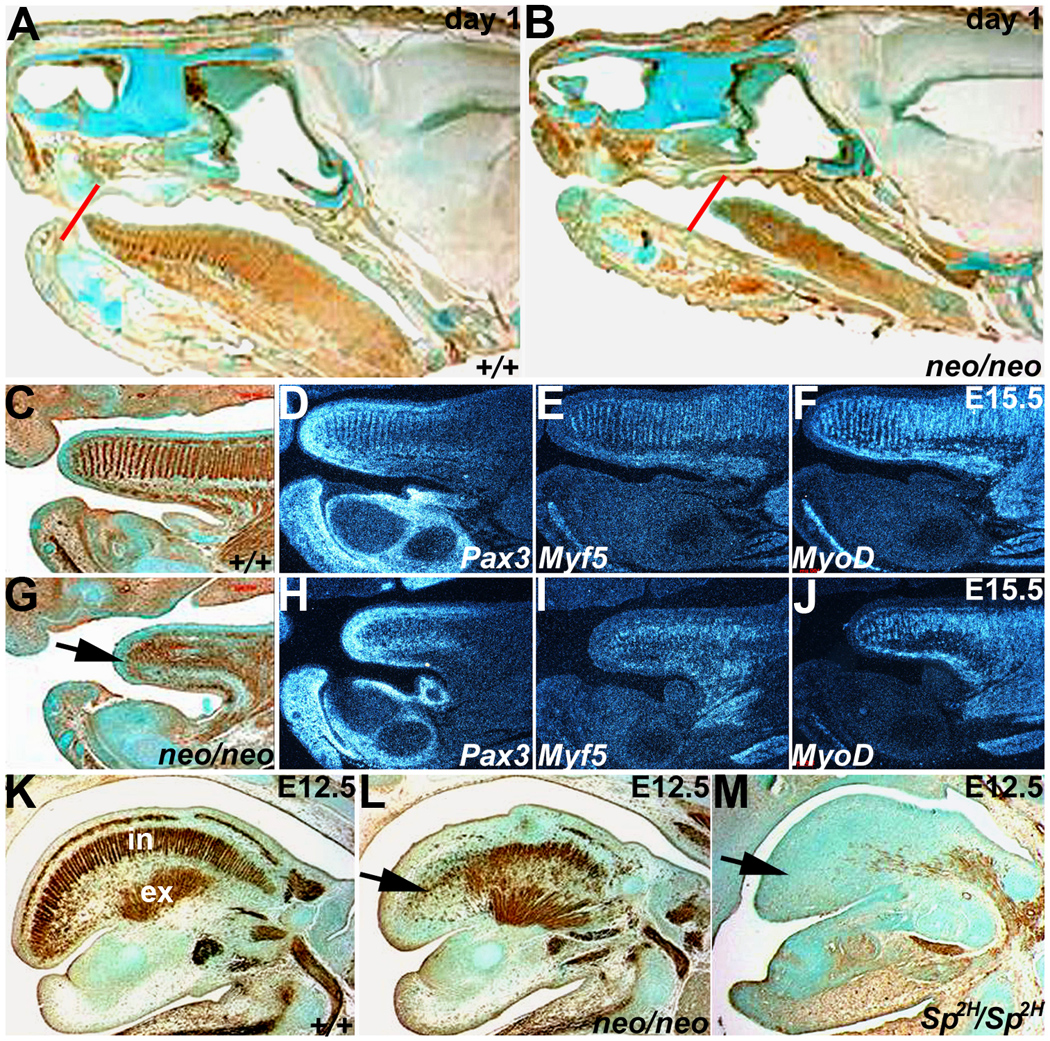
Both immunohistochemistry for alpha smooth muscle actin (αSMA) and radioactive in situ hybridization of Pax3 and early (Myf5) and late (MyoD) muscle marker expression were used to assess muscle deficiencies and pathogenesis of the anomalies at day 1 (A,B), E15.5 (C–J) and E12.5 (K–M). Day-1 sagittal sections stained for αSMA expression indicates that the Pax3neo/neo (B) tongue is shorter and thinner (red lines indicates the position of tongue tips) than in wildtype (A). (C&G) αSMA staining in E15.5 wildtype (C) and Pax3neo/neo (G) sagittal sections. Note E15.5 Pax3neo/neo tongue musculature was greatly reduced and disorganized. Pax3 (D,H), Myf5 (E,I) and MyoD (F, J) mRNA expression patterns mirror the disorganized αSMA-expressing cells on adjacent sections (C&G). The arrow in G points to location of tongue tip in Pax3neo/neo. Note the shorter tongue is already evident at this stage. (K-M) αSMA staining of sagittal E12.5 wildtype (K), Pax3neo/neo (L) and Sp2H/Sp2H (M) sections. Intrinsic (IN) muscles are partially absent whilst extrinsinc (EX) muscles are intact in Pax3neo/neo. However, both intrinsic and extrinsic Sp2H/Sp2H tongue muscles are absent. Arrow indicates intrinsic muscles affected in E12.5 Pax3 mutants.
