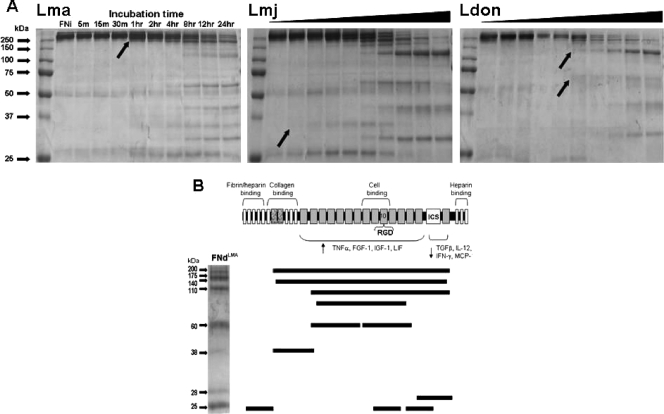
FIG. 3.
Time-dependent surface proteolytic degradation of FN by promastigotes of multiple Leishmania species (A) and mapping of the FN peptide fragments produced by L. amazonensis degradation (B). (A) Glu-fixed, stationary-phase promastigotes (107 in 100 ml PBS containing 10 μg FN at 25°C) of L. amazonensis (Lma), L. major (Lmj), and L. donovani (Ldon) were incubated for the indicated lengths of time (black triangles for Lmj and Ldon represent the same time course as shown for Lma), parasites were removed by microcentrifugation, and supernatants were processed for SDS-PAGE fractionation and Coomassie blue staining. L. major degraded FN the most rapidly (within 5 min), followed by L. amazonensis (1 h) and L. donovani (2 h), as indicated by the visualization of smaller FN proteins (black arrows). Each species produced a number of similarly sized protein fragments as well as some unique ones. (B) FNi was incubated with Glu-fixed L. amazonensis promastigotes for 24 h. FN fragments were separated by SDS-PAGE and stained with Coomassie blue. The visualized fragments shown were excised and submitted for mass spectroscopic analysis. The peptide sequences of each FN fragment were mapped according to their positions within the FNi. The functional domains of FN are indicated above the schematic, with the 10th repeat of the cell association domain containing the RGD sequence. The regions shown to activate (4) or deactivate (14) Mφs to produce different proteins are indicated below the schematic. TNF-α, tumor necrosis factor alpha; FGF-1, fibroblast growth factor 1; IGF-1, insulinlike growth factor 1; LIF, leukemia inhibitory factor; TGFβ, transforming growth factor β; IL-12, interleukin 12; IFN-γ, gamma interferon; MCP, monocyte chemoattractant protein.