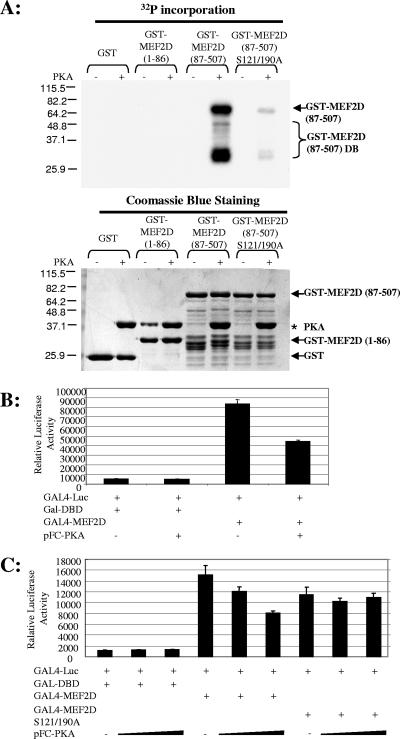
FIG. 6.
PKA targets MEF2D Ser 121 and Ser 190 to inhibit transcriptional activation properties. (A) Mutation of Ser 121/190 to alanine [GST-MEF2D (87-509) S121/190A] abolishes PKA-mediated phosphorylation compared to that of wild-type MEF2D [GST-MEF2D (87-509)]. Purified GST and GST-MEF2D (aa 1 to 86 or aa 87 to 507, wild type or S121/190A mutant) were incubated in vitro, with or without purified PKA, with [γ-32P]ATP, resolved using SDS-PAGE, visualized by Coomassie blue staining (lower panel), dried, and exposed to X-ray film for 30 min after the PKA kinase assay (top panel). These data are representative of two independent experiments. (B) C3H10T1/2 cells were cotransfected with GAL-DBD or GAL4-MEF2D (87-507), with or without pFC-PKA, and harvested 48 h after transfection. PKA suppressed MEF2D transcriptional activity. (C) C3H10T1/2 cells were transfected with GAL-DBD, wild-type GAL4-MEF2D (87-507), or mutated GAL4-MEF2D (87-507) S121/190A with increasing concentrations of pFC-PKA (0, 0.5, or 1 μg) and harvested 48 h after transfection. PKA suppressed wild-type GAL4-MEF2D (87-507) transcriptional activity but not that of the GAL4-MEF2D (87-507) S121/190A mutant, suggesting that the two PKA novel sites are responsible for PKA's negative regulation of MEF2D transactivation properties.