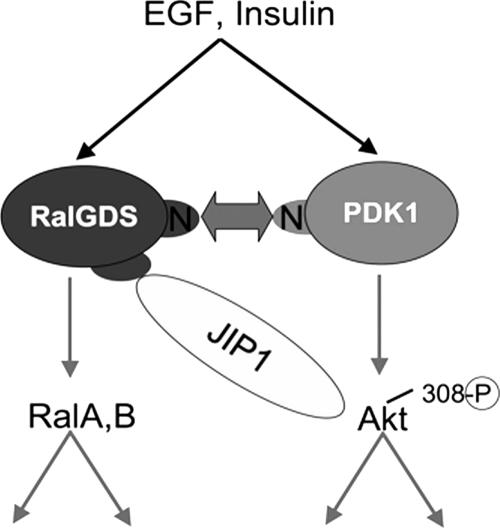
FIG. 9.
Two-way signal transduction between RalGDS and PDK1. Previously, we showed that PDK1 activates the intrinsic catalytic activity of RalGDS by forming a complex between its N terminus and the N terminus of RalGDS. Now we show that signal transduction travels in the other direction because RalGDS promotes the phosphorylation of Akt at T308 by PDK1 through the scaffold function of JIP1, which binds both Akt and RalGDS. The model suggests direct binding, but since these experiments were done in COS-7 cells it could be indirect. RalGDS also promotes the phosphorylation of Akt at S473, but the mechanism involved is different and not yet known. Thus, RalGDS transduces two important signaling cascades in response to growth factor stimulation, one that activates Ral GTPases through the exchange factor's catalytic domain and another that promotes Akt activation through the protein's scaffold function.