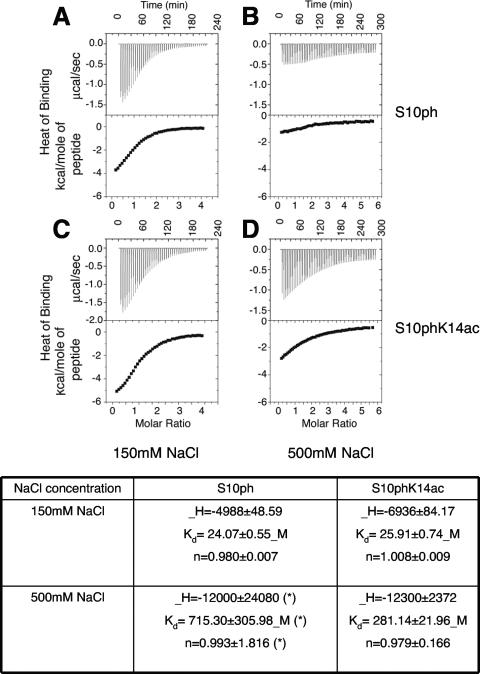
FIG. 5.
Isothermal titration calorimetry of Bmh1 binding to histone H3 peptides with different modifications. For each panel (A to D), the top panel shows the raw data for injections of the peptide into the Bmh1 solution as described in Materials and Methods; the lower section shows the integrated heats of injections, where panels A and B depict S10p binding at 150 mM and 500 mM NaCl, respectively, and panels C and D depict S10pK14ac binding at 150 mM and 500 mM NaCl, respectively. At the bottom of the figure, thermodynamic parameters involved in interaction of Bmh1 with modified histone H3 peptides are shown. The single binding site constant Kd and the heat of binding ΔH were used as fitting parameters in analysis of these data with the MicroCal software. The free energy and entropy changes for binding were then calculated by using the following relationships: ΔG = −RT ln Kd and ΔG = ΔH − TΔS, respectively, where R is the gas constant, T is temperature, and ΔS is the change in substrate concentration. In each case, parameters are reported with associated errors of the fit. The large error rate in S10Pho (500 mM NaCl) titration (indicated by an asterisk) shows that the binding is too weak to be detected.