FIG. 3.
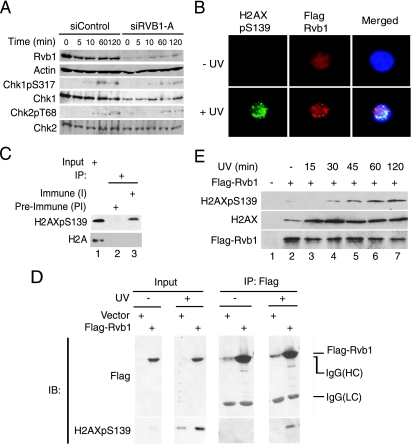
Rvb1 colocalizes and interacts with phospho-H2AX after DNA damage. (A) The loss of Rvb1 does not hyperactivate ATM and ATR kinases. Lysates from HCT116 cells were prepared after the transfection of the cells with the indicated siRNAs and the harvesting of the cells at the indicated time points post-UV irradiation. The results of immunoblotting with antibodies to Chk1 phosphorylated on S317 (Chk1pS317) and Chk phosphorylated on T68 (Chk2pT68) and anti-Chk1, anti-Chk2, anti-Rvb1, and anti-β-actin antibodies are shown. (B) Colocalization of Rvb1 with phospho-H2AX after DNA damage. 293T cells were transfected with a Flag-Rvb1-expressing plasmid. Cells were either left untreated (− UV) or irradiated with UV (+ UV). Immunofluorescence analyses using anti-Flag (red) and anti-phospho-H2AX (green) antibodies were performed. The merged images show the colocalization of Rvb1 and phospho-H2AX in DAPI-stained nuclei (blue). H2AXpS139, H2AX phosphorylated on S139. (C) Endogenous Rvb1 interacts with phospho-H2AX. The results of immunoprecipitation (IP) using either preimmune (PI) or immune (I) antibody against Rvb1 from lysates prepared from HeLa cells after UV irradiation are shown. Western blotting was carried out with anti-phospho-H2AX and anti-H2A antibodies. Ten percent inputs (lane 1) are shown in parallel. +, present. (D) Exogenous Rvb1 interacts with phospho-H2AX. 293T cells were transfected with a Flag-Rvb1-expressing plasmid. Cells were either left untreated (−) or irradiated with UV. Cell lysates were used to immunoprecipitate Flag-Rvb1 and subjected to Western blotting using anti-Flag and anti-phospho-H2AX antibodies. Results obtained using inputs of 10% of wild-type levels (input lanes) are shown in parallel. Input lanes of the immunoblot for H2AXpS139 represent a lighter exposure to see the stimulation of phospho-H2AX signals after UV treatment. IgG, immunoglobulin G; HC, heavy chain; LC, light chain; IB, immunoblot. (E) Increased interaction of Rvb1 with H2AX after DNA damage. 293T cells were transfected with an empty vector or a plasmid expressing FLAG-RVB1. Cells were irradiated and harvested at the indicated times after UV treatment. Lysates were immunoprecipitated with anti-Flag antibody, and immunoprecipitates were subjected to Western blotting for H2AX, phospho-H2AX, and Rvb1. +, present; −, absent.