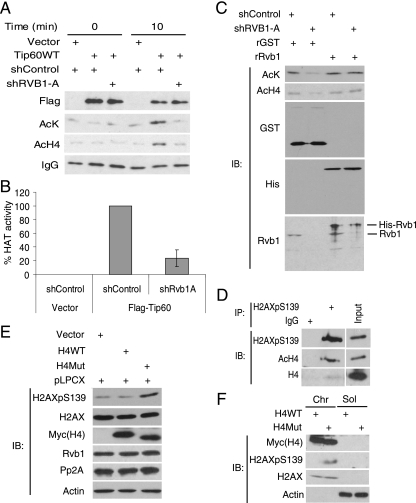
FIG. 7.
Rvb1 is required for the HAT activity of the Tip60 complex. (A) Rvb1 is required for the HAT activity of the Tip60 complex. 293T cells were transfected with a vector or a plasmid expressing FLAG-TIP60 together with a plasmid expressing an shRNA against RVB1 (shRVB1-A) or a control shRNA (shControl). The Tip60 complex was immunoprecipitated using anti-Flag antibody. Equal amounts of immune complexes were incubated for 0 or 10 min with core histones in the HAT assay. Acetylated histone H4 was detected by immunoblotting with anti-acetyl-lysine and anti-histone H4 antibodies. IgG, immunoglobulin G; AcK, acetyl-lysine; AcH4, acetyl histone H4; +, present. (B) Quantitation of HAT activity using anti-acetyl-lysine as measured in the analysis presented in panel A. Means ± SD of results from four experiments are shown. (C) Recombinant Rvb1 (rRvb1) restores the HAT activity of the Tip60 complex to cells depleted of Rvb1. The Flag-Tip60 complex was immunoprecipitated from cells transfected with the indicated plasmids and assayed for HAT activity as described for panel A. Either recombinant His6-Rvb1 (rRvb1) or nonspecific protein (recombinant glutathione S-transferase [rGST]) was added to the Tip60 complex as indicated. IB, immunoblot. (D) Phospho-H2AX coimmunoprecipitates with acetyl-histone H4. Anti-phospho-H2AX antibody was used for immunoprecipitation (IP) from lysates harvested 60 min post-UV irradiation. Immunoblotting was performed with the indicated antibodies. Ten percent inputs (input lane) are shown in parallel. H2AXpS139, H2AX phosphorylated on S139. (E) The expression of nonacetylable histone H4 increased total phospho-H2AX. 293T cells were transfected with the indicated plasmids, and cells were grown in the presence of puromycin for 48 h. Lysates were immunoblotted with anti-phospho-H2AX, anti-H2AX, anti-Myc (for wild-type and mutant histone H4 [H4WT and H4Mut]), anti-Rvb1, anti-PP2A, and anti-β-actin antibodies. pLPCX, plasmid containing the puromycin resistance gene. (F) Wild-type and nonacetylable histone H4 are incorporated equally into chromatin, but the nonacetylable H4 leads to an increase in the phosphorylation of chromatin-associated H2AX. The fractions were immunoblotted for the indicated proteins. The experiment was carried out as described for panel E. Chr, chromatin; Sol, soluble cellular extract separated from chromatin.