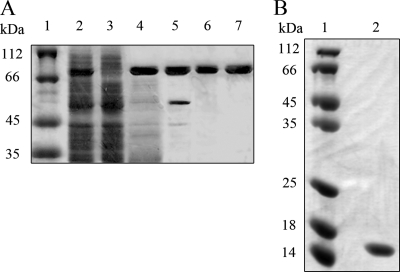
FIG. 1.
SDS-PAGE of StoHjm and StoHjc during purification. (A) Purification of StoHjm (81 kDa). Lanes: 1, molecular size markers; 2, total cell protein after sonication; 3, total cell protein of untransformed E. coli (as a control); 4, heat-treated supernatant (80°C, 30 min); 5, protein eluted from ion-exchange column; 6, protein after purification by nickel affinity chromatography; 7, protein purified by gel filtration. (B) Purification of StoHjc (14 kDa). StoHjc was purified by heat treatment, ammonium sulfate precipitation, anion exchange, Ni2+-nitrilotriacetic acid affinity chromatography, and gel filtration. Lanes: 1, molecular size markers; 2, purified StoHjc. All samples were separated on 15% denatured polyacrylamide gels. The gels were stained with Coomassie bright blue.