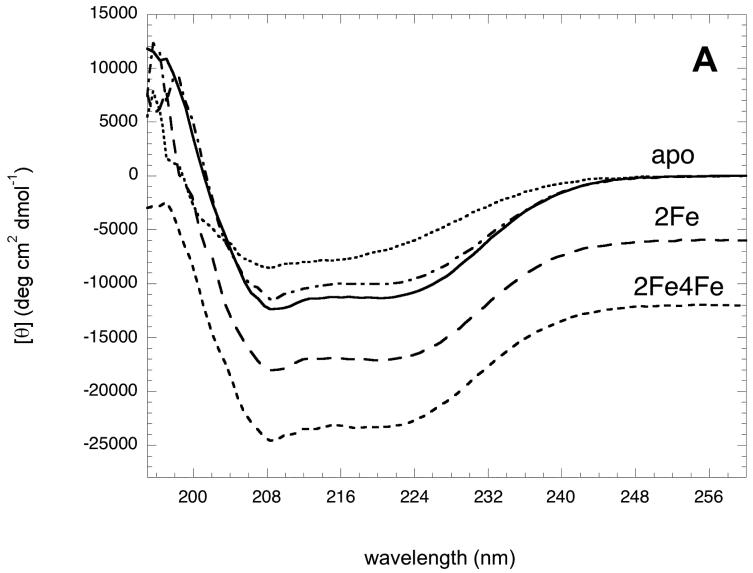
Figure 2.
Circular dichroism spectra of apoBioB, 2Fe-BioB, and 2Fe4Fe-BioB. (A) Circular dichroism spectra of 2Fe4Fe-BioB ( ), 2Fe-BioB (
), 2Fe-BioB ( ), and apoBioB (
), and apoBioB ( ) in 10 mM MOPS, 25 mM NaF, pH 7.5 at 25 °C. The spectrum of apoBioB is also shown at 4 °C (
) in 10 mM MOPS, 25 mM NaF, pH 7.5 at 25 °C. The spectrum of apoBioB is also shown at 4 °C ( ) and 95 °C (
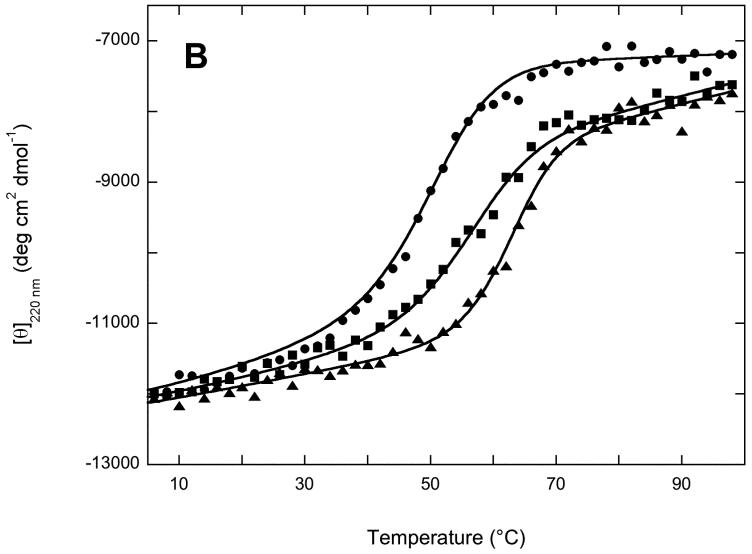
) and 95 °C ( ) (B) Thermal unfolding of 2Fe4Fe-BioB ( ▲ ), 2Fe-BioB ( ■ ), and apoBioB ( ● ) as monitored by circular dichroism at 220 nm. Each protein was incubated at 4 °C for 6 min, a spectral reading was taken, and the temperature was raised by 2 °C, repeating up to 98 °C. Attempts to measure a comparable curve for refolding during cooling proved unsuccessful due to aggregation of the unfolded protein. Data are fit to the Gibbs-Helmholtz equation as described in Materials and Methods.
) (B) Thermal unfolding of 2Fe4Fe-BioB ( ▲ ), 2Fe-BioB ( ■ ), and apoBioB ( ● ) as monitored by circular dichroism at 220 nm. Each protein was incubated at 4 °C for 6 min, a spectral reading was taken, and the temperature was raised by 2 °C, repeating up to 98 °C. Attempts to measure a comparable curve for refolding during cooling proved unsuccessful due to aggregation of the unfolded protein. Data are fit to the Gibbs-Helmholtz equation as described in Materials and Methods.