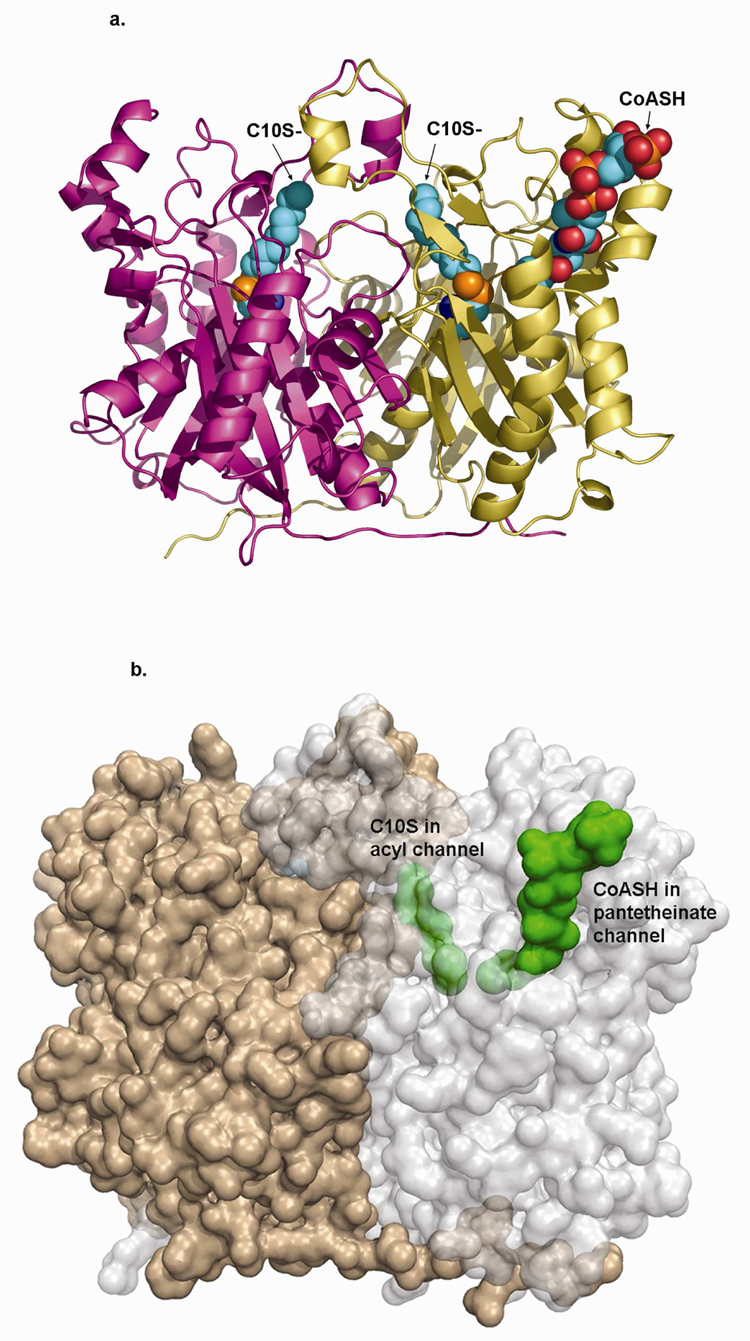
Figure 3.
a) Backbone structure of mtFabH homodimer (subunits in gold and magenta ribbon) in complex with C10S- (space filling) covalently linked to the sidechain sulfur of Cys112 (also space filling) in both subunits. CoASH is shown bound in only the A-subunit (gold), also in space filling model.
b) Semi-transparent surface figure of the mtFabH homodimer rotated slightly about the x-axis relative to A. Subunits are gold and light gray, C10S- and CoASH are green space filling and shown bound in only one subunit. The C10S- group in the acyl channel is completely buried (partly obscured by semi-transparent protein surface envelope); the distal end of the CoASH pantetheinate is buried, while the adenosine pyrophosphate end is exposed at the mouth of the pantetheinate binding channel.