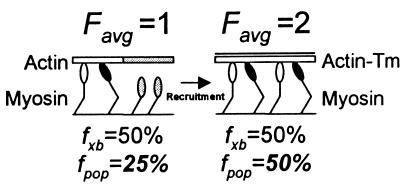
Figure 6.
Illustration of how a 2× increase in Favg (an ensemble force measurement), resulting from a change in XB recruitment due to the presence of Tm on actin, could be misinterpreted as a change in XB kinetics. In this case, we assume that the total XB population (n) equals 4 and that the XB duty cycle (fxb) is constant at 50% and unchanged in the presence of Tm. In addition, weakly bound XBs are depicted with open heads and strongly bound force-generating XBs are depicted with solid heads and contribute 1 unit of force. With actin alone (Left), it is modeled that the conformation of actin (shaded actin segment) prevents half of the XBs from attaching (shaded detached XBs). Given that only two XBs can interact with actin, and with fxb = 50%, then one XB will generate an Favg =1. However, if one estimates fxb based on Favg and n, then the population-based estimate of fxb is fpop = Favg/n or 25%. With the addition of Tm (Right), all XBs are capable of interacting with actin and Favg = 2. Even though fxb is unchanged, the population-based estimate has increased with fpop = 50%. Without knowing the true XB duty cycle, one could misconstrue the 2× increase in the population-based duty cycle estimate as an effect of Tm on XB kinetics rather than on recruitment. Thus, single molecule studies in the laser trap may be needed to definitively determine the contributions of changes in XB recruitment and kinetics to the observed effects of Tm on actomyosin mechanics.