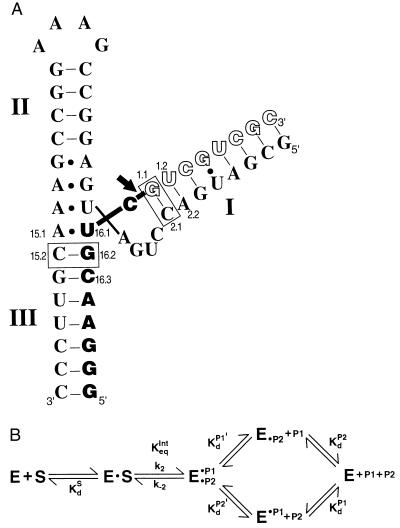
Figure 1.
The hammerhead ribozyme HH16 (A) and the kinetic and thermodynamic framework for its reaction (B). (A) The ribozyme is depicted with bound full-length substrate, S (empty and filled block letters). The arrow depicts the cleavage site, such that the filled block letters correspond to the 5′ product, P1, and the empty block letters correspond to the 3′ product, P2. The three helical regions and residues referred to in the text are numbered in accord with standard hammerhead nomenclature (6). The disposition of these helices and interactions of residues in the conserved core follow schematically from the x-ray crystal structure of complexes of the ribozyme with oligonucleotide substrate analogs (7, 8). The dashes refer to Watson–Crick base pairs, and dots refer to noncanonical base pairs observed. The ribozyme⋅substrate base pairs bordering the conserved core that give the large energetic effects discussed herein are boxed. (B) The reaction steps for the hammerhead ribozyme reaction (9). The ribozyme (E) cleaves its substrate, S, to give a 5′ product P1 with a 2′,3′-cyclic phosphate and a 3′ product P2 with a 5′-hydroxyl group. P1 and P2 are bound in helices III and I, respectively, as shown in A for S.