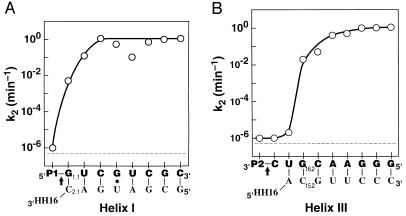
Figure 2.
Effect of individual substrate residues on cleavage by HH16. The rate constant for cleavage of bound substrate, k2, is shown for a series of oligonucleotide substrates with nucleotides added either to the 3′-end of P1 (A) or to the 5′-end of P2 (B). (That is, the point above the leftmost G in part A represents the rate constant for reaction of P1-G, and the point that follows above the U represents the rate constant for reaction of P1-GU. Analogously, the point above the leftmost C in part B represents the rate constant for reaction of C-P2, and the point immediately to its right represents the rate constant for reaction of UC-P2.) The arrow demarks the cleavage site. The rate constants were obtained in 50 mM Tris⋅HCl, pH 7.5/10 mM MgCl2 at 25°C as described in Methods, except for the rate constant above P1 in part A; P1 does include the cleavage site, so this rate constant was taken from the cleavage rate with oligonucleotides with mismatches in helix I: P1-A and P1-C (Table 1; note that P1-U may be slightly faster due to formation of a wobble pair). The broken line represents the background cleavage rate of 5 × 10−7 min−1. For P1-GUCGU, k2 is less than that for substrates shorter or longer by one residue; this decrease has been attributed to nonproductive binding of this oligonucleotide in an alternative duplex with HH16 (14).