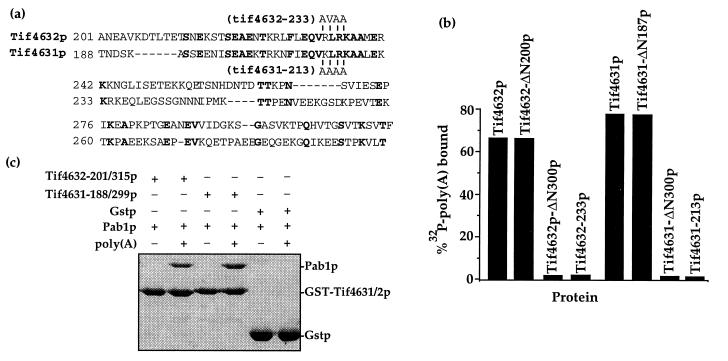
Figure 1.
Identification and mutagenesis of a Pab1p binding site on eIF4G. (a) Alignment of the Pab1p-binding domain of Tif4632p (6) and the homologous region of Tif4631p. Identical residues are highlighted, the positions and type of substitutions in Tif4632–233p and Tif4631–213p are indicated above or below the sequences, and the positions of the residues in the full-length proteins are shown. (b) Mutations within the Tif4631p and Tif4632p Pab1p binding domains destroy Pab1p binding. Each eIF4G protein containing the indicated mutation was fused to glutathione S-transferase, immobilized on glutathione resin, and assayed for its ability to bind to a [32P]poly(A)/Pab1p complex, as previously described (6). [32P]poly(A) retention reflects the amount of Pab1p binding to the eIF4G proteins. (c) The Pab1p-binding domain of Tif4631p resides between amino acids 188 and 299. The Tif4631p and Tif4632p fragments diagrammed in a were fused to glutathione S-transferase, immobilized on glutathione resin, and assayed for their ability to bind Pab1p in the presence or absence of poly(A), as previously described (6). Bound proteins were resolved in a 10% SDS/PAGE gel and visualized by Coomasie blue staining. The position of each recombinant protein is indicated.