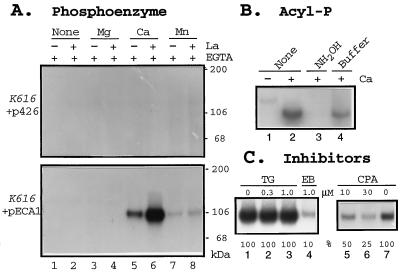
Figure 4.
Formation of a Ca2+-dependent phosphoprotein is inhibited by CPA. (A) Ca2+-dependent PE. Membranes were isolated from triple mutants, K616, transformed with either pECA1 (Lower) or with vector alone (Upper). Membranes were incubated with 2 nM [32P]ATP for 2 min with 0.5 mM EGTA alone (none), or with added divalent cations to give a final free concentration of 990 μM Mg2+, 45 μM Ca2+, or 3 μM Mn2+ in the presence (+) or absence (−) of 50 μM La3+. The reaction was stopped with TCA, and the proteins were analyzed by SDS/PAGE and autoradiography. (B) Sensitivity of PE to hydroxylamine. Membranes were isolated from pECA1-transformed triple mutants. PE formed without La3+ was terminated with TCA (as in A). The TCA pellet was either untreated (lanes 1 and 2) or incubated with 1 ml of 0.5M hydroxylamine (NH2OH) in 100 mM Mes-KOH buffer at pH 6.0 (lane 3) or with buffer alone (lane 4) for 15 min. Protein was precipitated with 0.2 ml 50% TCA. (C) Inhibition of PE formation by erythrosin B (EB) and CPA, but not by thapsigargin (TG). Membranes isolated from triple mutant transformed with pECA1 were incubated with DMSO (lanes 1 and 7) or inhibitors for 20 min before assay. PE formation was assayed with 45 μM Ca2+, and 100 nM ATP for 15 sec without La3+. PE was quantitated by PhosphorImager.