FIG. 1.
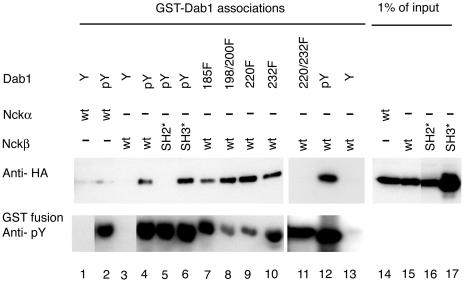
Dab1 tyrosine phosphorylation and the Nckβ SH2 domain are required for complex formation. Nck proteins were detected by anti-HA Western blotting (upper panel) of cell lysates from HEK293T cells transfected with Nckα (lanes 1, 2, and 14), Nckβ (lanes 3, 4, 7 to 13, and 15), Nckβ-R312K carrying a mutation in the SH2 domain (lanes 5 and 16), or Nckβ-W39,149,235K, which has mutations in all three SH3 domains (lanes 6 and 17). The proteins that bound the various GST-Dab1 fusion proteins were eluted by phenyl phosphate-containing buffer (lanes 4 to 17; upper panel). The GST-Dab1 fusions included Dab1-wt (lanes 1 to 6, 12, and 13), Dab1-Y185F (lane 7), Dab1-Y198,200F (lane 8), Dab1 Y220F (lane 9), Dab1 Y232F (lane 10), and Dab1 Y220,232F (lane 11) in their unphosphorylated state (Y; lanes 1, 3, and 13) or after tyrosine phosphorylation by Abl (pY or mutant designation, lanes 2 and 4 to 12). All GST fusion proteins incubated with Abl kinase were tyrosine phosphorylated as determined by Western blotting with antiphosphotyrosine antibody 4G10 (lower panel). Lanes 14 to 17 represent 1% of the amount of cell lysate used in the association assay (lanes 1 to 13).