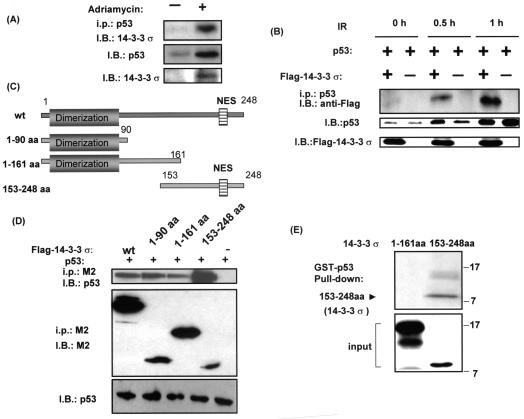
FIG. 1.
14-3-3σ interacted with p53. (A) Interaction between 14-3-3σ and p53 after adriamycin treatment. A549 cells were treated with 0.2 μg of adriamycin per ml (+) or not (−) for 24 h. Equal amounts of cell lysates were immunoblotted (I.B.) with anti-p53 antibody or anti-14-3-3σ to show the expression of p53 or 14-3-3σ after adriamycin treatment. Equal amounts of cell lysates were also immunoprecipitated (i.p.) with anti-p53 antibody, then resolved in SDS-polyacrylamide gel electrophoresis, and immunoblotted with anti-14-3-3σ antibody to observe the association between p53 and endogenous 14-3-3σ. (B) Interaction between 14-3-3σ and p53 after ionizing radiation (IR). R1B/L17 cells were transfected with the indicated Flag-tagged 14-3-3σ constructs and p53 expression vectors for 48 h and irradiated with 10 Gy. After the indicated hours of ionizing radiation, equal amounts of cell lysates were immunoprecipitated with anti-p53 antibody, and the immunoprecipitates were immunoblotted with anti-Flag antibody (M2) to observe the association between p53 and Flag-tagged 14-3-3σ. Cell lysates were also immunoblotted (I.B.) with anti-p53 antibody or anti-14-3-3σ to show the expression of p53 or 14-3-3σ. (C) Schematic representation of 14-3-3σ deletion constructs. (D) Interaction between the domains of 14-3-3σ and p53. R1B/L17 cells were transfected with the indicated Flag-tagged 14-3-3σ constructs and p53 expression vectors. Cell lysates were immunoprecipitated (i.p.) with anti-Flag antibody (M2), and the immunoprecipitates were immunoblotted (I.B.) with anti-p53 (DO-1; Santa Cruz) to observe the interaction. The same blot was stripped and immunoblotted with anti-Flag antibody (M2, Sigma) to demonstrate the amount of 14-3-3σ deletion constructs expressed and immunoprecipitated. Equal amounts of protein from the cell lysates were immunoblotted with anti-p53 (DO-1) to indicate the expression of p53. (E) p53 specifically interacts with 14-3-3σ in vitro. GST-p53 immobilized on glutathione beads was incubated with in vitro-transcribed and translated 14-3-3σ domains (amino acids 1 to 161 or 153 to 248), which were 35S labeled. Bound domains were detected by autoradiography. The bottom panel shows 10% of the in vitro-translated 35S-labeled 14-3-3σ domain inputs.